Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Preclinical data (Stim-R tech) https://www.nature.com/articles/s41598-024-72392-1
LYEL..................................https://stockcharts.com/h-sc/ui?s=LYEL&p=W&b=5&g=0&id=p86431144783
(OT) Dr Restifo is Co-founder and Chief Scientist at Marble Therapeutics. From this: ''At Marble Therapeutics, he is working to figure out how to rewind the epigenetic clock on these cells, using any means possible. The company started out with a focus on skin rejuvenation, adds CEO Denitsa Milanova, but expanded to also work on adoptive cell therapy to prove their platform works.'' https://web.archive.org/web/20231218233715/https://www.nature.com/articles/d41573-023-00206-6
They have a license for TIL plus a neoantigen vaccine https://www.federalregister.gov/documents/2024/02/07/2024-02491/prospective-grant-of-an-exclusive-patent-license-vaccine-augmented-adoptive-cell-therapy-for-the
Preclinical data on the vaccine https://jitc.bmj.com/content/11/Suppl_1/A418
LYEL.....................................https://stockcharts.com/h-sc/ui?s=LYEL&p=W&b=5&g=0&id=p86431144783
Tempting but I will remain on the sidelines.
They could be working on this https://ash.confex.com/ash/2023/webprogram/Paper182417.html
Preclinical data from another group (LYL119 will be NR4A3-deficient) https://www.biorxiv.org/content/10.1101/2023.04.21.537841v1.full
LYEL..........................https://stockcharts.com/h-sc/ui?s=LYEL&p=W&b=5&g=0&id=p86431144783
The FDA has cleared an IND for LYL845. The PhI trial will initially enroll patients with relapsed and/or refractory metastatic or locally advanced melanoma and subsequently expand into NSCLC and CRC. Initial data presentation is expected in 2024.
SITC titles
NR4A3 gene editing and c-Jun overexpression synergize to limit exhaustion and enhance functional activity of ROR1 CAR T cells in vitro and in vivo
Engineering potent CAR T-cell therapies by controlling T-cell activation signaling parameters using the Stim-R™ technology, a programmable synthetic cell-signaling platform
The Epi-R™ technology produces a polyclonal TIL product (LYL845) with diverse tumor-reactive clones that have stem-like qualities and anti-tumor function
The Epi-R™ technology produces a polyclonal TIL product (LYL845) with a greater expansion success rate across hot and cold tumors, improved product phenotype, and maintenance of TCR diversity
Increased potency and functional persistence in vitro of a next-generation NY-ESO-1-specific TCR therapy incorporating Gen-R™ genetic reprogramming technology
recent technical advances using culture conditions with IL-7/IL-15 and
the addition of IL-21 may enhance the enrichment for TSCM cells
in the final CART product (74–76), which can be further increased
with the addition of drugs blocking T-cell differentiation, such as
glycogen synthase-3 inhibitors (77). Nevertheless, robust clinical grade protocols for generating TSCM-enriched CART products
have not been developed so far. Recently, a few CART19 clinical
trials for DLBCL have been conducted in which CART products
were manufactured from CD62L+ isolated T cells to generate
cellular products enriched for TCM cells (21, 78); however, due to
prolonged culture conditions, enrichment for TSCM and TCM
subsets in the infused product could not be demonstrated
https://www.frontiersin.org/articles/10.3389/fimmu.2022.904497/full
The AEs from the LD chemo*. I expect more data (dose expansion is ongoing) from them at ASH. As for NVS, a pivotal trial is planned and DL2 (12.5M) is the recommended dose.
* Some very early clinical data testing an anti-GD2 CAR-T with constitutive signaling from an engineered IL-7 receptor
Thanks for the link.
Most commonly reported grade 3/4 AEs were neutrophil count decrease (6/9, 67%), anemia (5/9, 56%), thrombocytopenia (2/9,22%), platelet count decrease (2/9, 22%) and no infection was reported. Complete response rate(CRR) was 78%
Compared to T-Charge
http://www.koreabiomed.com/news/articleView.html?idxno=12767
For Epi-R, they use media with high concentrations of potassium (as well as multiple cytokines). As for that, T-Charge or BE, I think a product with mostly ''stem-like'' cells and additional edits will be needed. But it doesn't have to be the latter, as shRNA can be used. Here are some clinical data on an anti-CD19 CAR-T with knock-down of both PD-1 and TIGIT https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.7522
Which will perform better ? BE with 7,8 edits on many genes, Epi secret sauce
or T-Charge which seems to add a CAR only to starting material in order to retain CD4/CD8 ratio.
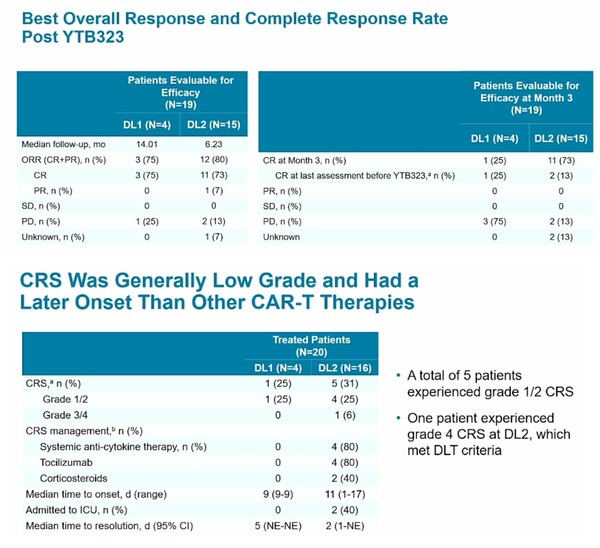
YTB323 CAR-T cell products generated via this novel expansionless manufacturing process retained the immunophenotype of the input leukapheresis materia
That depends. Typically, the more the cells expand during manufacturing, the more they differentiate. In the case of ALLO, it seems most are effector memory (manufacturing time is ~19 days and there are a number of steps involved). LYEL refer to this as the expansion/quality paradox. With Epi-R, they have shown they can expand TCR-T cells up to 18 billion by day ten. In addition, these cells are over 94% viable and maintain their ''stem-like'' qualities.
Do you need Epigenetic Reprogramming if pts can be treated with healthy, young donor T cells?
1115: Epigenetic Reprogramming (Epi-R™) Yields T-Cell Receptor Products with Improved Stemness, Metabolic Fitness, and Functional Activity in the Presence of Persistent Antigen Exposure https://annualmeeting.asgct.org/abstracts/abstract-details?abstractId=2014
661: Preclinical Development of LYL797, a ROR1-Targeted CAR T-Cell Therapy Enhanced with Genetic and Epigenetic Reprogramming for Solid Tumors https://annualmeeting.asgct.org/abstracts/abstract-details?abstractId=1597
GSK has updated the master protocol for their next generation constructs to include a third arm with GSK4427296 https://clinicaltrials.gov/ct2/show/NCT04526509
This uses Epi-R.
The PhI (n=54) of LYL797 has now been listed. The dose-escalation phase (TNBC only) will investigate four dose levels to determine the recommended RP2D. The dose expansion will enroll both TNBC and NSCLC.
Morgan Stanley webcast https://morganstanley.webcasts.com/viewer/event.jsp?ei=1488969&tp_key=3cedc4a917
|
Followers
|
0
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
32
|
|
Created
|
06/18/21
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
