Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
I'm looking forward to how prospective partners may use lenzilumab. For instance, I see that Novavax states they, "Demonstrated our variant strain change capabilities, updating our vaccine to the XBB.1.5 variant for the 2023-2024 vaccination season." Was their ability to accommodate variant strain changes accomplished by enhancing their vaccine with our variant-agnostic lenz?
see pg 4/162 "COVID-19 Clinical and Strain Change"
https://www.sec.gov/Archives/edgar/data/1000694/000100069424000026/novavax2023annualreport-fi.pdf
Also, I'm particularly interested in what role lenz could play in treating or preventing malaria.
"Based on the conclusions reached in the aforementioned studies, we gather that cytokines are important modulators of the immune response in malaria. Dysregulation of the cytokine network in severe malaria is linked to parasite and host factor variations. We have found that many of the cytokines involved in malaria (TNF-a, IFN-?, IL-4, and IL-10) play a double role, as a friend or as an enemy. Proinflammatory cytokines control parasite multiplication and promote parasite clearance. However, elevated levels of proinflammatory cytokines such as TNF-a, IL-6, or IL-8 may be markers of severe malaria. TGF-ß is probably the most important regulatory cytokine that limits the inflammatory process in malaria. Maintaining a balance between proinflammatory and anti-inflammatory cytokines is essential, and disrupting this molecular harmony can lead to unfavorable disease evolution. A better understanding on the cytokine's involvement in malaria pathogenesis could provide the basis for the discovery of novel diagnostic markers and indicators of disease progression and severity, as well as the foundation for the development of new malaria vaccines."
See the Conclusions:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8592744/#:~:text=Proinflammatory%20cytokines%20control%20parasite%20multiplication,the%20inflammatory%20process%20in%20malaria.
Prospective partner Sanofi's Global Health Unit plans to, "...provide access to a broad portfolio of medicines in 40 countries with the highest unmet medical needs. To that end the GHU created Impact, a unique not-for-profit brand with 30 standard-of-care medicines produced by Sanofi, some of which are considered essential by the World Health Organization (WHO). The Impact medicines cover a wide range of therapeutic areas including diabetes, cardiovascular disease, tuberculosis, malaria and cancer."
See pg 11, "Corporate Social Responsibility update at the end of the Q1 2024"
https://www.sec.gov/Archives/edgar/data/1121404/000112140424000011/pressreleaseq12024english.htm
Sanofi's product pipeline also includes objectives for treating other indications, such as asthma, which could also be an application for lenz.
I think these are exciting times for Humanigen, and I look forward to my open sell order being filled at $200, in the short squeeze we will have when management recalls their loaned shares.
Jay lost his entire "investment" in a pennyscam. Again.
SPNGQ
HGENQ
The company received 2. million in licensing revenue. Taran with their stalking bid bought those 2 2 million dollar receivables and the cash receivables from hgenq so that 2 million they are just buying the cash and accounts receivables for their $2 million dollar bid and get the ip patents for nothing. as the asset put the ip patent as zero value.
Without the fake 37 million in lawsuit claim by pathogen and chime and company spending over 10 million in legal fees in 3 years. this coompany is not bankrupt with only 4 employees, it raised over 100 million in ipo financing assuming that was true and no fake ipo money. The only money the company made could have been shorting shares in ipo to public investors..who how much money was raised in the nasdaq sold to public investors as naked short positions and still not covere>? or shorted that is unknown.
why you need to courts permission for payment or anything? waste of time submitting all the forms need court approval
HGENQ gets a $50,000 'settlement' from EMD lawsuit for breach of contract, the same lawsuit by chime and pathogen that was seeking $37 million and the $5 million 'settlement' that cause the company to go bankrupt after FDA rejected the LENZI third stage clinical application.
it was " NOT" the REJECTION by the FDA that caused chapter 11 but the lawsuits afterwards that caused chapter 11 and inability to raise additional capital from new investors and shareholder or the controlling shareholder wanted to go bankrupty to embezzle the patents in sham auction.
nobody would pay more than $50,000 or even $10,000 for cancellation of a contract in business. it was just RFQ to the vendor and no contract s were signed with chime or pathognen and no services were even rendered.



LENZI which was bought for nothing to hgenq shareholders. Now in business, if assets are so below 'fair value' it can be void and buyer knows it's below fair value. I don't know what law that voids transactions. but if an asset was sold to cheap and sold to insiders it considere 'embezzlement' which is why all gov't assets are sold in an OPEN public auction, and anyone with cash can bid on it. In this case of LENZI auction it was NOT an open bid auction. and people were not allowed to bid on it. so it was NOT sold at 'fair price' and seller didn't actively solicit bidders, and based on the conditions, it discouraged any bidder or didn't respond to interested bidders.
In most 'auction's the price of the asset is already known, before the auction is held. and bidders just participate and pay 'fair price'
These drug patents are worth a lot of money if it sells as insurance companies are paying for these treatments 3.5 million/treatment. Who would have that kind of money if they don't have the 3.5 million and no insurance?
these are rare diseases and only a few pay for them.
The treatment will be available by prescription to eligible patients this quarter, a Pfizer spokesperson told CNBC. It has a hefty $3.5 million price tag before insurance and other rebates, the spokesperson added, making it by far one of the most expensive drugs in the U.S.
https://www.cnbc.com/2024/04/26/fda-approves-pfizer-gene-therapy-beqvez-for-treatment-of-hemophilia-b.html
Speaking of Durrant's Amendment to the Milestone Events, Novavax also filed an Amendment to their Articles of Incorporation.
https://www.sec.gov/Archives/edgar/data/1000694/000100069424000013/0001000694-24-000013-index.htm
And Sanofi really seems to be getting things ready for their spinoff.
https://www.sec.gov/Archives/edgar/data/1121404/000112140424000011/pressreleaseq12024english.htm
I think either, or both, of these companies represent potential partnerships for Humanigen.
your were sold hopium and your drank their coolaid.
there was no intention of selling a legitimate investment opportunity or building a real business.
going bankrupcty and stealing investor equity and embezzling corporate assets was the 'evil plan'
As they say, this 'crew' is really good.
you've been creamed by the grand masters of investment fraud.
What don't you understand Cowtownjay, shareholders of hgenq no longer own any asset other than promissory note and that is even being stolen if the hgenq is dissolved. What don't you understand? Accept the facts. there is no hope based on the status quo or information we have now. it's very clear in the chapter 11. they planned this entire fraud operation. like master con artist that is how good they are.
Welcome to the room, and to Ihub, TimelessOasis72. Don't pay any attention to the emojis.
I'm sorry that I can't answer your question. I opted out of the class action. But I would be the first to join a lawsuit against the class action lawyers.
Besides, I think lenz may be demonstrating just how widely it will be accepted into the market, well beyond the covid and oncology indications, where lenz has clinically proven its first-in-class safety and efficacy. I'm glad to see this success being factored into a more rapid and expanded amended version of the Milestone Events. I think that's a truly exciting development, yet it gets absolutely no recognition of its significance by shareholders. I'm sure, though, that investor sentiment will improve sharply, once details of our opportunity are spelled out, letter-by-letter, by management.
Thanks for posting!
phantom trades, phantom of the market.
These trades are NOT HUMAN!! You, you, you are not human!
Some human turned the machines back on this stock
The autobots are still here and back.
It seems the autobots are BACK,,which is good, at totally abandon stock, even autobots are shut down
insurance policy like this buying your wife $10 million dollar life insurance without here knowing about it and a year later your wife died from natural causes and you get the $10 million dollar life insurance and you only bought it one year ago.
The company only agree to pay the $3 million so they can claim the $5 million managmeent liability insurance JACKPOT payout. The company only paid $200,000 in insurance premiums in 2022 and was sued in same year?
could be never because, the lawsuit claim of $3 million was not even on the list of creditor claims in the chapter 11 'petition'. Its a pre petition 'settlement' not a conviction or verdict, so legally its has weight. in law of court.
The law firm Claim #10013
Value $3,000,000.00
Creditor SCOTT GREENBAUM AND JOSHUA MAILEY
Filed Apr 04 2024
and supposedly had the $3 million in insurance money that was paid out the management liability insurance. it was a settlement so it's up to them to pay. a settlement doesn't mean they are guilty or even forced to pay later. both sides agree to 'settle' and can always go to court 'again' if one doesn't honor their word. but the court didn't order the payment.
a settlement is not the same as court order or defendent is guilty of anything. if you they don't pay there is nothing you can do about it and since it's so low you probably don't want to waste months for $200 pay day. 3 million -1.5 mill legal fee. -= 1.5 million in pot/ 1000 claimants it's only $1,500 even 100 people it's only $15,000
How long until you think we’ll actually see the payout on our claims?
The corruption of the FDA is that a drug approval or not approval is about the money and corruption has always been about the money. but the corruption goes too far when people die because of corruption like FDA approving drugs like oxycotin and doctors prescribing it knowing it was bad for drug abuse for profit etc or not approving certain drugs because of competitor don't want it approve or bribed not to approve it. corruption in gov't was taking bribes to approve certain projects and in many countries corruption was expected to get the gov't to do anything to subsidize low wages of gov't workers. and was a gift. or tip. and expected to pay. like SEC approving crypto ETF and exchanges listing obviously fake fraudulent financial instruments.
the FDA or any or when the gov't is corrupt, they no longer have the mandate of heaven and lose authority. that is how gov't and dictators fall, gov't corruption. Gadafffi in Libya embezzled gov't assets to himself and same with Saddam huessien, they stole the state's money for his person account. and lived like Kings were not Kings. but dictators and eventually they were hanged by the people and his personal gaurds who were bribed to kill him.
In North Korea, they perfected state propaganda, Kim is like god or hero to the people. The imperial family in many countries were they were divine family and it's authority was the King was the son of God. or the King was the ultimate shaman. so that is how the King had power and the King's word is law.
“Repeat a lie often enough and it becomes the truth”, is a law of propaganda often attributed to the Nazi Joseph Goebbels. Among psychologists something like this known as the "illusion of truth" effect..--- gov't (FDA) propaganda.
The time was, when talk about waning was taken for granted to refer to a vaccine's efficacy over even short periods of time. Now, waning refers to the body's immune response, in terms of less effective antibodies being produced. But, are there any serious proponents advocating for a third mRNA jab, especially in light of all the serious adverse events apparently resulting from a third jab?
The only serious option is to use lenz in lieu of a third mRNA jab.
"Exploratory analysis for the effect of lenzilumab on SWOV (Survival Without Ventilation, primary endpoint) was conducted by the CRP baseline quartile. Response to lenzilumab was observed in the first through third quartiles of baseline CRP with the greatest lenzilumab treatment effect observed in the first quartile (CRP <41 mg/L; HR: 8.20; 95% CI 1.74 to 38.69; p=0.0079).
see pg 3/11, next to the last paragraph:
https://thorax.bmj.com/content/thoraxjnl/78/6/606.full.pdf
with all those shares you have you can do whatever you want pontious.
The address of this company has changed too. so why is it still paying $1336/month for a mail box in some business centre in some office share building?
I wonder about that? I don’t understand the incessant posting, but the facts are there and validated by someone going through the filings almost line by line.
Pontious Pilot doesn’t know who to crucify at this point.
Clinical stage 3 is the last FDA stage before approval for commercialization.
The question is the LENZI molecule even work or effective in commercial use for blood cancer or preventing corvid virus immune system boost? this drug like many drugs have many uses. one is blood cancer another is boosting immune system.
anyways. the company has no intention of getting approval and could have sabotage their FDA submission so they don't get approved. why pay catalent, biowa, any money because they are doing work on the LENZ molecule and Taran if they want their services have to pay. Taran owns the 90% of hgenq shares that is why they are not selling. the company use third party companies to do all the work and manufacturing of the drug. and it's tcompanies like biowa, pathogen, catalents who do all the paperwork for submission to FDA. the company is just owner of the lenzi patent and has no other assets. the employees and board of directors of the company or liabilities and why are they still not fired. and still paying $40,000/month to two useless Board of directors for doing nothing of value. but instead ripping existing shareholder value and embezzling corporate assets.
ronald barliant
rainer boehm
who are just board of directors were paid over $40,000 for doing nothing of value
they have other jobs and this job is plum job for doing nothing but writing checks to the lawyers.
these two guys are just hire hands and front man to give the impression they represent the shareholders of interest of hgenq but from what I see they are part of the plan to embezzle assets to taran and liquidate assets of the corporation and rip off the ipo investors who were sold ove 150 million in shares.
they don't even have to be paid for anything as there is no business to run or manage in chapter 11
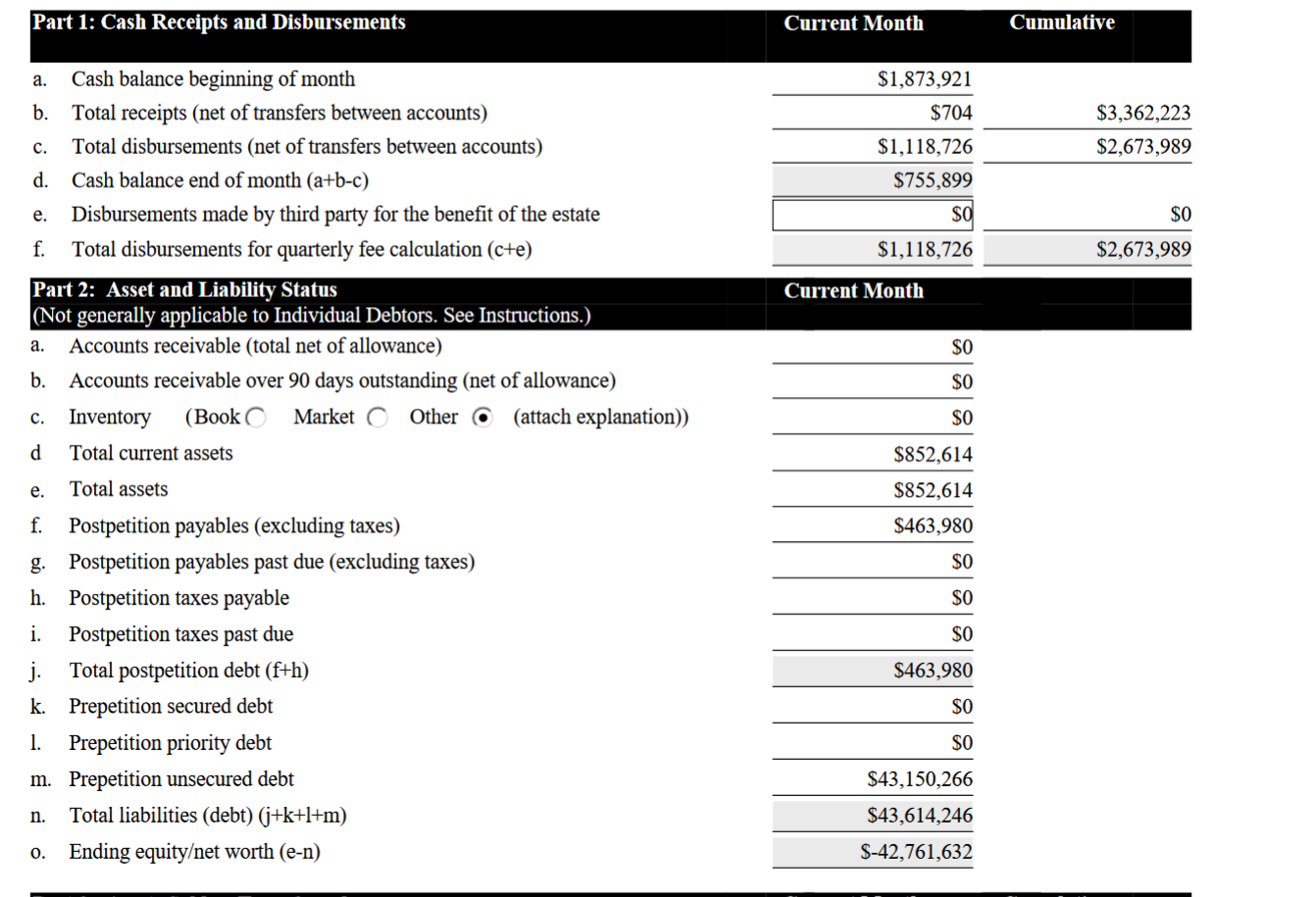
Before the bankruptcy humanigen had over 5 million in cash and equivalent assets not including the ip patents and only 2 million in liabilities. That 5 million in cash and equivalent didn't include the ip lenz patent tht was generating 2.5 million licensing fees just from Austalian subsidiary.. of the 44.10 million in fake creditor claims only 1-2 million was real debt owed. the rest was fraudulent creditor claims. as no company would give credit that much and no bank will lend money to humanigen as they had no collateral and no revenue. The company could have just went to the market to raise additional investment and didn't need the bank loans. so no bank would lend money to humanigen as an unsecured lender.
The Chime and and pathogen just two 'disputed' claim which was breach of contract lawsuit was like 37 million dollars of the 44.10 million 'creditor claim and because of the lawsuits the company was not able to raise capital by issueing any equity as most biotech would do. but that was the plan. to go bankrupt and wipe out existing ipo investor equity. by auction assets in sham auction that was sold for nothing in rigged fraudulent sham auction.
The plan is to dry and bleed every dollar out of this company before dissolving the company.
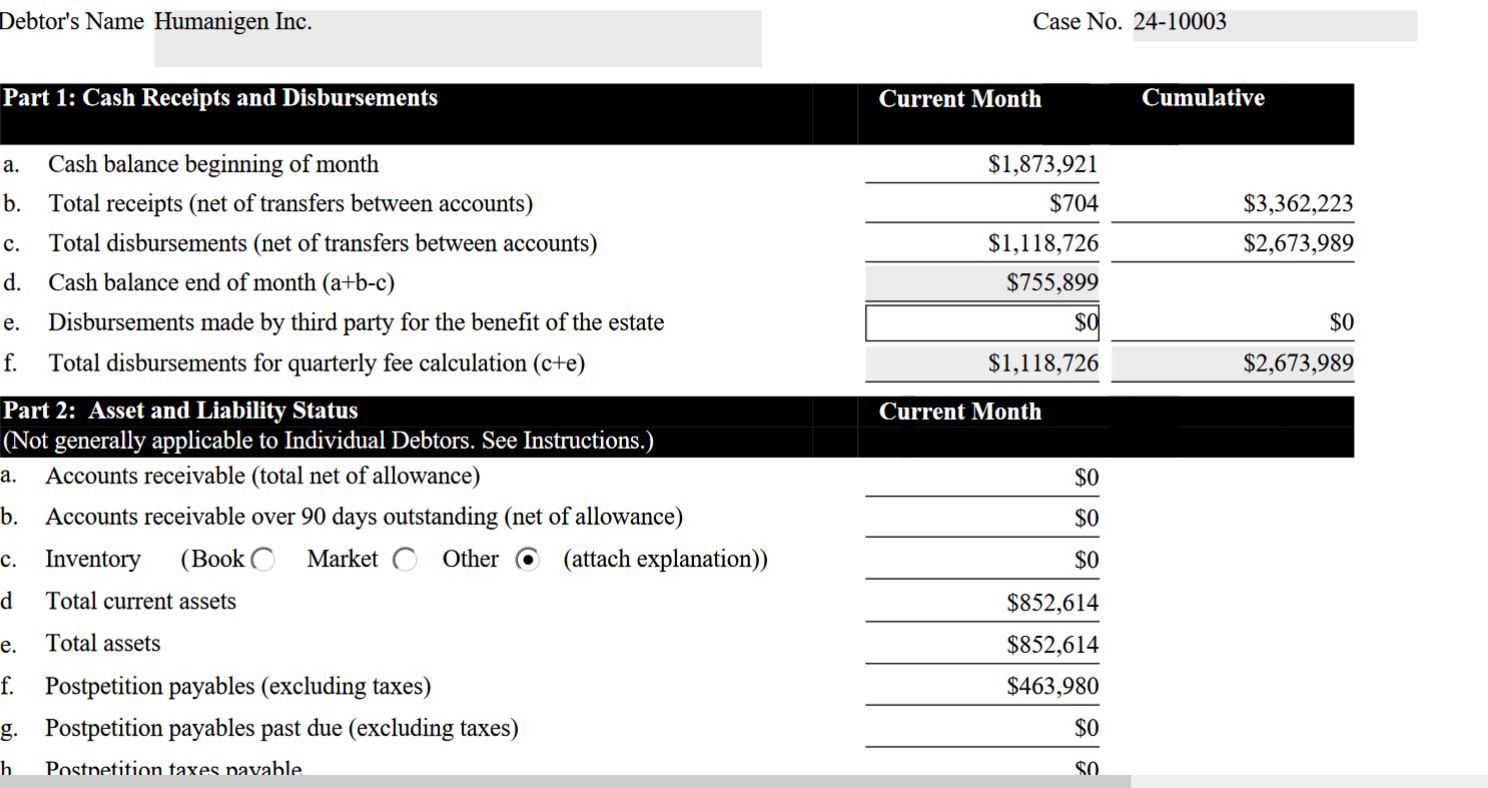
As of march 31 thee was a total of $755,899 cash balance.
you cannot dissolve a company with that much assets. As for the $463,980 post bankruptcy debt the 40 million pre bankruptcy debt was fake so is the $463,980 debt. why pay fraudulent creditor claims?
Why is this company still wiring over $400,000 to Biowa and Catalent? its in chapter 11 so it doesn't have to pay any of the creditors. Are those companies own by Taran? and same with over 1 million in legal fees for chapter 11. Chapter 11 is basic legal process, there is no need for all this paper work.
some bot had verbal diarrhea today. relax it will be back to 0 tomorrow
Technical BREAKOUT it closed at .0067? on no volume 2000 shares is only $13.40? who is messing around ?
gap from .0001 to .0067 no sellers between .0001 and .0067 that is how illiquid this stock is. and some autobot is still short 39,987 shares. as for march 28 , 2024?
Was this some an autobot or human?
What is going on with $HGENQ. I would appreciate if someone makes me a resume.
another crime is the insurance fraud, the company bought the $5 million dollar management liability insurance from some company and paid over $100,000/year in premiums// only fraudulent companies would buy that insurance. they know they well be sued or bought insurance and commit some crimes and get sued and claim the insurance payment for management being sued. none of the management being sued is even fired.
insurance fraud, they wanted to get sued. why anyone want to sell those management liability insurance. because these lawsuits can go unlimited high and that is the problem where management intentionally does some investor fraud and wants get sued so go bankrupt.
as for the insurance companies, they are making big bucks in premiums as the people buying is the corporation not the individuals. essentially management is not personal liable for investor fraud or mismanagement.
before filing for bankruptcy chapter in 2022, the company received over 2.5 million australian licensing for the lenzi patents. FDA is just for US market, other countries have their regulatory approval. and hgenq license to Australia they could have made millions in licensing if australia drug regulatory agency approves it. but since the patents are sold for 'free' to taran who bought it for free. hgenq gets nothing. and its now being dissolved. basically, this is classic case stock fraud. and embezzlement of corporate assets via fake chapter 11 or bankrutpcy fraud, and creditor claim fraud.
With secure creditors like bondholders, they can seize assets of the company if you miss just one payment or break loan convenants ie . getting delisted from stock market, sales fall below revenues, this bankruptcy was voluntary by hgen controlling shareholder, it didnt' have to file for chapter 11 because there is nothing the unsecured creditors can do. the 3 million dollar payment was not a ' conviction' by the juror that the company was liable. HGENQ management settled and agreed to pay the company cash of 3 million to the lawsuit because it bought 'insurance' for $5 million. the company paid close to $500,000 in insurance premiums and got paid $5 million in insurance pay out.so it settled. 2 million for legal fees and 3 million to the .047share settlement on condition - legal fees.
hgenq has NO SECURE creditors, and according to the plan it needs no approval from any non-secure creditor to dissolve the company. The decision to dissolve the company is solely on the entity that controls 90% of hgenq shares which is why there is no shares for sale under .01/cent.
"I don't get this need creditor approval to liquidate hgenq for what?"
Friday's filing by Durrant of Exhibit A was in regards to the, "...monetizing and distributing contingent assets such as the Milestone Payments..." This amendment obviously reflects a belief that either Durrant, the Creditors, or all of them, anticipate either a BLA or foreign regulatory approval of lenz. That's what it would take to monetize the Milestone Payments.
Again:
https://document.epiq11.com/document/getdocumentsbydocket/?docketId=1075977&projectCode=HUM&docketNumber=235&source=DM
this is an abandon stock, abandon market. untradeable ticker in many tickers.
you know it's bad when even the Autobots have abandon this stock and not trading it. zero volume nothing on the ask and nothing on the bid.
all the autobots have abandon this stock too.
only an autobot would short 100 shares at .0001 or trade 100 shares of a .0015 stock.
you know that there is ZERO volume, nobody selling below .01/share
yet one entity owns 110 million shares. why is that guy who owns 110 million shares not selling his worthless shares?
there is a 1 million dollar sell order at .01 cent.
lol , management has about 15% of shares
dtg and pontious pilot have as many
|
Followers
|
326
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
43436
|
|
Created
|
01/31/13
|
Type
|
Free
|
| Moderators cowtown jay | |||


Humanigen, Inc. is a clinical-stage biopharmaceutical company developing its portfolio of next-generation cell and gene therapies for the treatment of cancers via its novel, GM-CSF neutralization and gene-knockout platforms. As a leader in GM-CSF pathway science, we believe that we have the ability to transform CAR-T therapy and a broad range of other T-cell engaging therapies, including both autologous and allogeneic cell transplantation. There is a direct correlation between the efficacy of CAR-T therapy and the incidence of life-threatening toxicities (referred to as the efficacy/toxicity linkage). We believe that our GM-CSF neutralization and gene-editing platform technologies have the potential to reduce the inflammatory cascade associated with serious and potentially life-threatening CAR-T therapy-related side effects while preserving and potentially improving the efficacy of the CAR-T therapy itself, thereby breaking the efficacy/toxicity linkage. Clinical correlative analysis and pre-clinical in vivo evidence points to GM-CSF as the key initiator of the inflammatory cascade resulting in CAR-T therapy’s side-effects. Pre-clinical in vivo data on the neutralization of GM-CSF using antibody or gene KO indicates that it is not required for CAR-T cell activity. Our strategy is to continue to pioneer the use of GM-CSF neutralization and GM-CSF gene knockout technologies to improve efficacy and prevent or significantly reduce the serious side-effects associated with CAR-T therapy.
We believe that our GM-CSF pathway science, assets and expertise create two technology platforms to usher in next-generation CAR-T therapies. Lenzilumab, our proprietary Humaneered® anti-GM-CSF immunotherapy, has the potential to be used in combination with any FDA-approved or development stage CAR-T therapy, as well as in combination with other cell therapies such as HSCT, to make these treatments safer and more effective. In addition, our GM-CSF knockout gene-editing platform has the potential to create next-generation CAR-T therapies that may inherently avoid any efficacy/toxicity linkage, thereby potentially preserving the benefits of the CAR-T therapy while altogether avoiding its serious and potentially life-threatening side-effects.
The company’s immediate focus is combining FDA-approved and development stage CAR-T therapies with lenzilumab, the company’s proprietary Humaneered® anti-human-GM-CSF immunotherapy, which is its lead product candidate. A clinical collaboration with Kite, a Gilead Company, was recently announced to evaluate the use of lenzilumab with Yescarta®, axicabtagene ciloleucel, in a multicenter clinical trial in adults with relapsed or refractory large B-cell lymphoma. The company is also focused on creating next-generation combinatory gene-edited CAR-T therapies using strategies to improve efficacy while employing GM-CSF gene knockout technologies to control toxicity. The company is also developing its own portfolio of proprietary first-in-class EphA3-CAR-T for various solid cancers and EMR1-CAR-T for various eosinophilic disorders. The company is also exploring the effectiveness of its GM-CSF neutralization technologies (either through the use of lenzilumab as a neutralizing antibody or through GM-CSF gene knockout) in combination with other CAR-T, T cell engaging, and immunotherapy treatments to break the efficacy/toxicity linkage including the prevention and/or treatment graft-versus-host disease (GvHD) in patients undergoing allogeneic HSCT. The company has established several partnerships with leading institutions to advance its innovative cell and gene therapy pipeline.
June 15, 2020
Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Hospitalized Patients With COVID-19 Pneumonia
https://clinicaltrials.gov/ct2/show/NCT04351152
Anti-GM-CSF antibodies expected to show better effect in Covid-19 than cytokine-specific targets
July 27, 2020
https://discoverysedge.mayo.edu/2021/06/22/cancer-to-covid-19/

| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
