Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Good luck explaining your actions where you are headed Mr Varner?
IMO
$GNPX About Genprex, Inc.
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex's technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex's oncology program utilizes its systemic, non-viral Oncoprex® Delivery System which encapsulates the gene-expressing plasmids using lipid-based nanoparticles in a lipoplex form. The resultant product is administered intravenously, where it is taken up by tumor cells that then express tumor suppressor proteins that were deficient in the tumor. The Company's lead product candidate, Reqorsa® Immunogene Therapy (quaratusugene ozeplasmid), is being evaluated in three clinical trials as a treatment for NSCLC and SCLC. Each of Genprex's three lung cancer clinical programs has received a Fast Track Designation from the FDA for the treatment of that patient population, and Genprex's SCLC program has received an FDA Orphan Drug Designation. Genprex's diabetes gene therapy approach is comprised of a novel infusion process that uses an AAV vector to deliver Pdx1 and MafA genes directly to the pancreas. In models of Type 1 diabetes, GPX-002 transforms alpha cells in the pancreas into functional beta-like cells, which can produce insulin but may be distinct enough from beta cells to evade the body's immune system. In a similar approach, GPX-002 for Type 2 diabetes, where autoimmunity is not at play, is believed to rejuvenate and replenish exhausted beta cells.
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by visiting the Company Website, registering for Email Alerts and by following Genprex on Twitter, Facebook and LinkedIn.
$GNPX New CEO Mr. Varner co-founded Genprex in 2009, and he has served on the Company's board of directors since 2012. He has served as President and CEO of Genprex since 2016. Under Mr. Varner's leadership, Genprex became a publicly traded company in 2018 and has successfully completed two Phase 1 clinical trials, opened three additional clinical trials, expanded its intellectual property portfolio, exclusively licensed new novel gene therapy technologies and has received three U.S. Food and Drug Administration Fast Track Designations and one Orphan Drug Designation.
$GNPX announced Ryan Confer Appointed Genprex President and CEO and to its Board of Directors.
AUSTIN, Texas, May 8, 2024 /PRNewswire/ -- Genprex, Inc. ("Genprex" or the "Company") (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced with great respect and sorrow the unexpected passing last night of the Company's co-founder, President, Chairman and Chief Executive Officer (CEO), Rodney Varner, due to sudden complications after a courageous battle with cancer.
$GNPX News: Genprex Announces the Passing of its Co-Founder and Chief Executive Officer, Rodney Varner https://finance.yahoo.com/news/genprex-announces-passing-co-founder-220800772.html?soc_src=social-sh&soc_trk=tw&tsrc=twtr via @YahooFinance
Rinse and repeat? GNPX a mechanism to steal?
The only business they might be in is dilution?
Every single person associated with GNPX should maybe be in jail?
Whos pockets did the dilution go?
IMO
GNPX: effective Feb. 2,2024 a one for 40 reverse split:
https://hedgefollow.com/upcoming-stock-splits.php
Still can't understand why no one from Genprex is in jail?
From the absolute beginning when they hired Redman it was destined to steal?
IMO
The gap could be endless selling of shares raising money for anything other than research and development?
Is it theft?
Find where the money goes and shut it down?
IMO
missed offering disclosure... sold lol
Need to fill the gap @ .85. This is a pump from the Insider Financial folks. Be careful buying here, don't chase.
$GNPX - Up 6.8% Pre-Market/ Current Price $0.88
Receives Fast Track Designation From The FDA For Its Small Cell Lung Cancer Drug Candidate| 07/11/2023
What kind of news would propel this stock over $1?
No need to promote deceptive videos?
GNPX is diluting shares?
And that's it?
They have been fund raising dilution since inception and that is all they will do until bankruptcy?
These shell companies are thrown together by the same minds who have done this multiple times with other shells?
They take a shelved product, tweek it then dilute shares based off of false theories?
GNPX will go to pennies and shareholders will continue to lose?
But what happens to the hundreds of millions they steal?
Nothing ever happens?
IMO
$GNPX Interesting video.
https://www.genprex.com/videos/nasdaq-gnpx-is-advancing-cancer-and-diabetes-treatments/?
wabadon
I agree 100%. Been accumulating for a year.
GNPX
wabadon
THAT ONE IS FOR "INVESTMENT". BIG FISH DOWN THE ROAD, JUST MY ONE CENT OPINION
Why is no one in Genprex is jail for money laundering?
Where is all this money going?
Will a future bankruptcy whipe out years of share selling, bad hires and firings?
GNPX now sits at .88 heading to sub penny?
And all is good?
Keep your receipts?
IMO
Also Known As
John Rodney Varner, Rodney R Varner, Rodney Te Varner, Rodney A Varner, Jrodney Rodney Varner, Rodney Varner Ave
Imagine you could go through life with this many alias?
Genprex firing of Michael T Redman is not enough?
John Rodney Varner has so far produce nothing but dilution buying and selling shares of Genprex Inc?
Or Rodney Varner? Whatever the hell he calls himself today?
Then pays himself almost a million a year to do so?
Is it a scam money laundering felony to obtain a shell with the sole purpose of dilution shares and paying yourself?
How else is Rodney John Varner John Varner Rodney paying himself?
Profit?
Is none?
Jail time has to be the only outcome if John aka Rodney aka JVarner is involved in faking studies to sell shares?
And judging by the raising O/S and his pay so far that's about all he is producing?
Am I wrong Johnathan? Johnny?
IMO
The SEC If allowing GNPX to hide the blatant dilution and not require them to report the actual O/S is blatant corruption?
This could be just another share selling fund raising scheme?
Total fraud?
Should go bankrupt like Redmond did with Oncolix?
Genprex Inc followed Redmans script then tossed him out?
IMO
Familiarize yourselves with pump and dumps?
IMO
Take a look at the chart and understand what this group can be doing?
Taking shelved failed products, changing, recirculating them while selling shares?
Never wanting to hint at an actual viable product?
This could be a pure scheme to enrich many at the expense if shareholders?
The Execs at this company have done this before with other failed companies and might continue until MD Anderson and the rest of them get brought up on possible charges?
IMO
3300 BEE CAVE ROAD, #650-227 Is a mail center?
Mail Center USA?
The man who configures his name differently based on different reports does business out of a mail center?
J Rodney Rodney J Varner J?
Whatever?
Fraud?
IMO
Why did Varner John Rodney dipose 1.6 million shares?
Thats one hell of a disposal?
GNPX is the garbage disposal?
IMO
Looking like another low volume manipulation pump day.
Looking good
Pls go all the way back in my posts… I belobe there is Even a Option to see
YES I was in buddy
With still being over bought, it could be setting up in this now bad market for a selloff below $2.00.
Fake Investors of GNPX, since the DD has been researched on this shell, what's the current O/S?
Not the one from last November, today's?
I would assume investing into a company it's current numbers would be something that would be needed to know?
Maybe not?
IMO
Who wants to bet if today a green or red day? The responses will be accepted before market open only. Do not respond after market opens!
Tomorrow sounds like round 2 for a run…
Man, you sound angry
Tomorrow we may have another run…wait to see what tomorrow brings…
Complete Bullshit!
You were not?
IMO
Not in… I was in the run a couple years ago from 0,60-7$ … but started selling above 5-7
Was amazing
Yeah better wait for opening bell…

Our technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options.
We are developing gene therapies for patients with cancer and diabetes to bring new therapies to large patient populations. Our lead product candidate, Reqorsa® Immunogene Therapy, is designed to be administered in combination with other therapies, including targeted therapies and immunotherapies, for non-small cell lung cancer and small cell lung cancer.
We have assembled a multidisciplinary team of executives and advisors with broad business experience in the biotech and pharmaceutical industries, and research and clinical experience at preeminent medical and academic institutions around the world. Our management team pairs an unmatched expertise with a knack for innovation in research.
At Genprex, we are committed to developing life-changing gene therapies for patients afflicted with cancer and diabetes. We are dedicated in our efforts to helping patients with limited or no treatment options to have access to new and advanced therapies to more effectively treat their disease.
Ryan Confer Appointed Genprex President and CEO and to its Board of Directors
AUSTIN, Texas — (May. 1, 2024) — Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced the Company’s participation in the following upcoming investor and industry conferences to be held in May 2024.
Compelling Data Validates the Potential of Reqorsa® Immunogene Therapy and the Oncoprex® Delivery System as Innovative Cancer Treatments
Multiple clinical trial sites to be opened under collaboration with large network of community-based oncology practices
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex’s technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex’s oncology program utilizes its proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, which the Company believes is the first systemic gene therapy delivery platform used for cancer in humans. ONCOPREX encapsulates the gene-expressing plasmids using lipid nanoparticles. The resultant product is administered intravenously, where it is then taken up by tumor cells that express tumor suppressor proteins that are deficient in the body. The Company’s lead product candidate, REQORSA™ (quaratusugene ozeplasmid), is being evaluated as a treatment for non-small cell lung cancer (NSCLC) (with each of these clinical programs receiving a Fast Track Designation from the Food and Drug Administration) and for small cell lung cancer. Genprex’s diabetes gene therapy approach is comprised of a novel infusion process that uses an endoscope and an adeno-associated virus (AAV) vector to deliver Pdx1 and MafA genes to the pancreas. In models of Type 1 diabetes, the genes express proteins that transform alpha cells in the pancreas into functional beta-like cells, which can produce insulin but are distinct enough from beta cells to evade the body’s immune system. In Type 2 diabetes, where autoimmunity is not at play, it is believed that exhausted beta cells are also rejuvenated and replenished.
We are committed to fighting cancer and diabetes by continuing to develop unique and innovative gene therapies.
Our pioneering technologies are at the forefront of gene therapy and we stand behind our high-quality technology platform.
We have integrity and high ethical standards, and we believe in responsibly transforming the lives of those affected with cancer and diabetes.
Our commitment, excellence in technology, and integrity behind what we do enables us to be trailblazers in the effort to change the lives of patients afflicted by cancer and diabetes.
We have assembled a multidisciplinary team of executives and advisors with broad business experience in the biotech and pharmaceutical industries, and research and clinical experience at preeminent medical and academic institutions around the world. Our management team pairs an unmatched expertise with a knack for innovation in research.
Our lead drug candidate, REQORSA® Immunogene Therapy (quaratusugene ozeplasmid) for non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), is designed to interrupt cell signaling pathways that cause replication and proliferation of cancer cells, target and kill cancer cells, and stimulate the natural immune responses against cancer. REQORSA is an immunogene therapy in that it combines features of gene therapy and immunotherapy. It up-regulates TUSC2 expression in the cell, and also increases the anti-tumor immune cell population and down-regulates PD-L1, thereby potentially boosting the immune response to cancer.
In 2020, the FDA granted Fast Track Designation for REQORSA in combination with AstraZeneca’s Tagrisso® (osimertinib) in late-stage NSCLC patients with EFGR mutations whose tumors progressed after treatment with Tagrisso. In 2021, the FDA granted Fast Track Designation for REQORSA in combination with Merck & Co’s Keytruda® (pembrolizumab) in late-stage NSCLC patients whose disease progressed after treatment with Keytruda. In 2023, the FDA granted Fast Track Designation for REQORSA in combination with Genentech, Inc.’s Tecentriq® in patients with extensive-stage small cell lung cancer (ES-SCLC) who did not develop tumor progression after receiving Tecentriq and chemotherapy as initial standard treatment. The FDA also granted Orphan Drug Designation for REQORSA for the treatment of SCLC.
REQORSA consists of the TUSC2 gene expressing plasmid encapsulated in non-viral nanoparticles made from lipid molecules (our ONCOPREX® Nanoparticle Delivery System) with a positive electrical charge. REQORSA is injected intravenously and specifically targets cancer cells, which generally have a negative electrical charge. REQORSA is designed to deliver the functioning TUSC2 gene to cancer cells while minimizing their uptake by normal tissue.
Tumor biopsy studies conducted at MD Anderson show that, in three patients, the uptake of TUSC2 in tumor cells after REQORSA treatment was 10 to 33 times the uptake in normal cells. We believe that REQORSA is the first systemic gene therapy to be used for cancer in humans.
Unlike many other gene therapies, REQORSA is administered intravenously and it does not need to integrate into the patient’s DNA. Many other gene therapies require complex procedures that result in permanent changes in a patient’s DNA, including the removal of cells from a patient and the modification of those cells which are then reinfused into the patient.
Multimodal Mechanism of Action
Many approved cancer therapeutics target only single molecules or a single specific genetic abnormality related to driving the proliferation and survival of cancer cells. In contrast, REQORSA has been shown to have a multimodal mechanism of action whereby it interrupts cell signaling pathways that cause replication and proliferation of cancer cells, re-establishes pathways for programmed cell death (apoptosis) in cancer cells, and modulates the immune response against cancer cells. REQORSA also has been shown to be complementary with targeted drugs and immunotherapies.

Overcoming Drug Resistance
Resistance to targeted drugs and checkpoint inhibitors develop through activation of alternate bypass pathways. For example, when PD-1 is blocked, the TIM-3 checkpoint is up-regulated. We believe that REQORSA’s multimodal activity will block emerging bypass pathways, thereby potentially reducing the probability that drug resistance develops.
Combination Therapies
Our preclinical and clinical data indicate that REQORSA is well tolerated and may be effective alone or in combination with targeted small molecule therapies. Preclinical data indicate that REQORSA may also be effective with immunotherapies, and in a three-drug combination with immunotherapy and chemotherapy. These data suggest that REQORSA, when combined with other therapies, may be effective in a large population of lung cancer patients.
To learn more about scientific evidence and studies supporting REQORSA and the TUSC2 gene, please refer to our Clinical Trials and TUSC2 Bibliography pages.
In diabetes, we have exclusively licensed from the University of Pittsburgh multiple technologies relating to the development of a gene therapy product for each of Type 1 and Type 2 diabetes.
The same general novel approach is used in each of Type 1 and Type 2 whereby an adeno-associated virus (AAV) vector containing the Pdx1 and MafA genes is administered directly into the pancreatic duct. In humans, this can be done with a routine endoscopy procedure. Our diabetes product candidates are currently being evaluated and optimized in preclinical studies at the University of Pittsburgh.
GPX-002 is designed to work in Type 1 diabetes by transforming alpha cells in the pancreas into functional beta-like cells, which can produce insulin but may be distinct enough from beta cells to evade the body’s immune system.

GPX-002 has been tested in vivo in mice and nonhuman primates. Earlier studies in diabetic mouse models showed that an earlier version of GPX-002 restored normal blood glucose levels for an extended period of time, which lasted approximately four months, and markedly increased the mass of insulin producing beta cells. According to the researchers, the duration of restored blood glucose levels in mice could potentially translate to decades in humans.
In Type 2 diabetes, GPX-003 is believed to work by replenishing and rejuvenating the beta cells that make insulin.
In August 2022, we entered into a one-year sponsored research agreement with the University of Pittsburgh for the use of GPX-003 in a non-human primate (NHP) model in Type 2 diabetes.
In February 2023, the Company’s research collaborators at the University of Pittsburgh presented preclinical data in a NHP model of Type 1 diabetes highlighting the therapeutic potential of GPX-002 at the 16th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2023) in Berlin, Germany. The statistically significant study results showed that after infusion of the AAV engineered construct all eight of the NHPs had:
We believe the data in NHPs demonstrate the potential for this gene therapy treatment to eliminate the need for insulin replacement therapy for Type 1 and Type 2 diabetic patients.
To learn more about scientific evidence and studies supporting GPX-002, GPX-003 and the Pdx1/MafA genes, please refer to our Clinical Trials and Pdx1/MafA Bibliography pages.
Our oncology platform utilizes our non-viral Oncoprex® Nanoparticle Delivery System. Using this system, anti-cancer genes expressing DNA plasmids contained in lipid nanoparticles are delivered intravenously to the patient. This platform, originally developed through collaborative research between the University of Texas MD Anderson Cancer Center and the National Institutes of Health, has been optimized to work with our initial drug candidate, Reqorsa® Immunogene Therapy (quaratusugene ozeplasmid).
REQORSA® utilizes the ONCOPREX® Nanoparticle Delivery System to encapsulate the TUSC2 gene in positively charged nanoparticles that bind to negatively charged cancer cells, and then enter the cancer cell through selective endocytosis, a process by which cells take in substances from outside the cell by engulfing them in a vesicle. The nanoparticles in our system differ significantly from liposomes historically used for drug delivery in that they are true particles encapsulating the therapeutic payload within a bilamellar lipid coat.
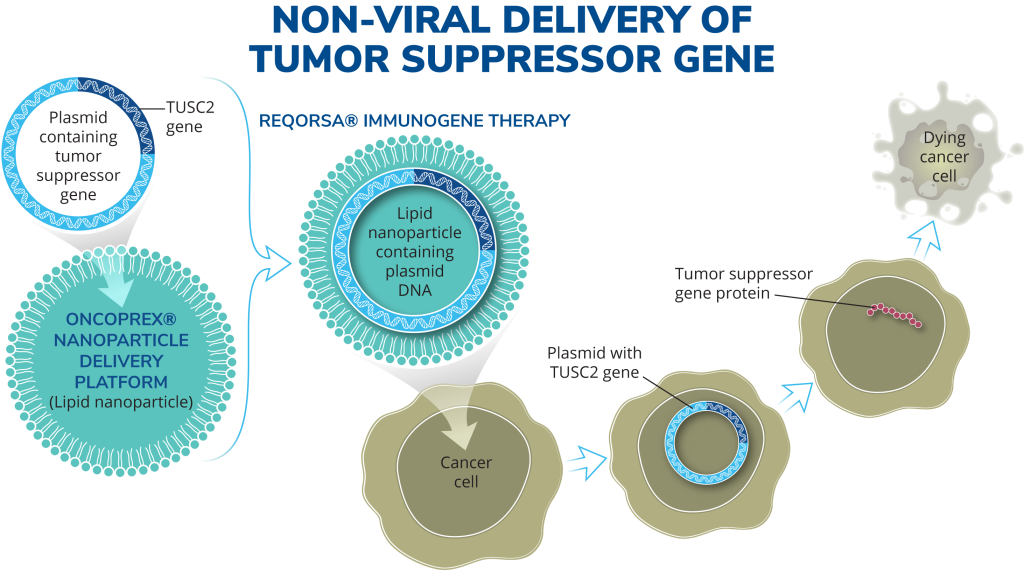
Our systemic, nanoparticle, non-viral delivery system, which is being used in our clinical trials for the treatment of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), is designed to be small enough to cross tight barriers in the lung, but large enough to avoid accumulation in the liver, spleen and kidney. The cationic (positive) charge of the nanoparticles target cancer cells. A Phase 1 clinical trial showed that intravenous REQORSA therapy selectively and preferentially targeted tumor cells, resulting in anticancer activity. The nanoparticles are non-immunogenic, allowing repetitive therapeutic dosing and providing extended half-life in the circulation.
In mice studies, the nanoparticles have been shown to be taken up by tumor cells after REQORSA administration at 10 to 33 times the rate they are taken up by normal cells.
We have administered REQORSA to more than 50 patients in Phase 1 and 2 clinical trials using our systemic, proprietary, ONCOPREX non-viral delivery system.
A Phase 1 clinical trial showed that systemic, intravenous REQORSA therapy using the ONCOPREX Nanoparticle Delivery System selectively and preferentially targeted tumor cells, resulting in clinically significant anticancer activity. The nanoparticles are non-immunogenic, allowing repetitive therapeutic dosing and providing extended half-life in the circulation.
Our earlier clinical trials have also shown that REQORSA therapy using the ONCOPREX Nanoparticle Delivery System is well tolerated in humans and can be delivered at high therapeutic doses. We believe the ONC-001 clinical trial was the first systemic gene therapy clinical trial using a nanoparticle delivery system to deliver a tumor suppressor gene.
Genprex’s Chief Medical Officer discusses the Company’s collaboration with a large network of community-based oncology practices to open additional sites for the Acclaim-3 clinical trial in SCLC
The KOL event, “Bringing Gene Therapy to the Fight Against Lung Cancers,” features discussions by Alexander I. Spira, MD, PhD, FACP; Daniel Morgensztern, MD; and Mark S. Berger, MD, Chief Medical Officer at Genprex, highlighting REQORSA® as a potential treatment for both NSCLC and SCLC.
Innovators with Jane King profiles tech companies and thought leaders in the space. Genprex (NASDAQ: GNPX): is focused on developing life-changing therapies for cancer & diabetes patients.
In this exclusive Global One Media interview, Genprex, Inc. (NASDAQ: GNPX) Chief Financial Officer, Ryan M. Confer, delves into Genprex’s groundbreaking work so far in clinical-stage gene therapy.
Video Highlights Validation of the ONCOPREX® Nanoparticle Delivery System with a Second Tumor Suppressor Gene
Watch Genprex’s KOL event, titled, “Groundbreaking Data From Preclinical Study Reported at ATTD 2023: Novel Gene Therapy To Treat Type 1 Diabetes.” This discussion is moderated by Genprex’s Chief Medical Officer, Mark Berger, M.D., and the data is presented by George Gittes, M.D., Professor of Surgery and Pediatrics and Chief of the Division of Pediatric Surgery at the University of Pittsburgh School of Medicine.
Genprex, Inc. CEO Rodney Varner tells Proactive the FDA has granted Fast Track Designation to the company’s lead drug candidate, REQORSA Immunogene Therapy, in combination with Merck & Co Inc (NYSE:MRK)’s Keytruda in patients with stage III or IV non-small cell lung cancer (NSCLC), where disease progressed after treatment with Keytruda.
Note: When you click on media articles below, a new page will open indicating that you are leaving the Genprex, Inc. website and entering a third-party website not affiliated with Genprex Inc. or any of its affiliates. No information contained in a linked site has been endorsed or approved by Genprex, Inc. and Genprex, Inc. is not responsible for the content of such third-party websites.
Genprex’s Chief Financial Officer, Ryan Confer, shares his insight on partnering with academic institutions, the benefits, and best practices
Diabetes is the most expensive chronic illness in the U.S., with $1 of every $4 in healthcare costs going to care for diabetics. The National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) estimates that $237 billion is spent directly on diabetes-related medical costs every year, while another $90 billion is lost each year due to reduced productivity associated with diabetes.
Bell2Bell’s latest podcast features Chairman, President and CEO Rodney Varner and CFO Ryan Confer of Genprex, Inc. (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients living with cancer and diabetes. In this interview, Varner and Confer discuss the journeys that led them to Genprex and the promise of the company’s drug development pipeline.
Bell2Bell’s latest podcast features Dr. Mark Berger, Chief Medical Officer of Genprex Inc. (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients living with cancer and diabetes.
On the heels of the release of recent positive safety and early efficacy data related to the phase 1 portion of its Acclaim-1 phase 1/2 clinical trial for non-small cell lung cancer evaluating REQORSA in combination with Tagrisso, Genprex has released a new patient video featuring an NSCLC patient from the phase 1 portion of its Acclaim-1.
Genprex Inc., a clinical-stage gene therapy company, is focused on developing for these patients with serious medical conditions and unmet need new and better treatment options that are life-changing.
Reprogramming the course of cancer and diabetes, Austin-based Genprex is on the cutting edge of technologies to address the biggest health concerns
Genprex, a clinical-stage gene therapy company, has recently released groundbreaking data from a non-human primate study evaluating a novel gene therapy to treat Type 1 diabetes.
With results of an animal study published in February 2023, Genprex aims to add credibility to another genome editing approach in the hunt for a functional cure to T1D.
How one company seeks to provide hope to cancer patients through more effective treatments.
DISCLAIMER:
Nothing in the contents transmitted on this board should be construed as an investment advisory, nor should it be used to make investment decisions.
There is no express or implied solicitation to buy or sell securities.
The author(s) may have positions in the stocks or financial relationships with the company or companies discussed and may trade in the stocks mentioned.
Readers are advised to conduct their own due diligence prior to considering buying or selling any stock. All information should be considered for information purposes only.
No stock exchange has approved or disapproved of the information here.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
