Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
still holding gltu
out before the dump, next.
wow really is some power in this hour look at those buys
trying to draw an inside straight here on the river.. volume is nutz..just an impulse shot here 1k shares .. friday action yikes
Premarket pump jobs always tank, 95% of the time, very risky
No news on anything their science is based on, simply a pump job here! Scrap imo
Nice LOL. Nice volume too. Let's see this back over the $. ![]() https://finviz.com/quote.ashx?t=SEEL&p=d
https://finviz.com/quote.ashx?t=SEEL&p=d
Shs Float 2.71M
From 10Q As of August 6, 2024, 5,956,903 shares of the common stock, par value $0.001, of the registrant were outstanding.
https://ih.advfn.com/stock-market/NASDAQ/seelos-therapeutics-SEEL/stock-news/94383074/form-10-q-quarterly-report-sections-13-or-15d
Not getting my hopes us they mega dumped here.
Let's see this follow SNGX ![]()
looking at me funny now
me too- i am eyeing it closely. Took starter.
Do you give a lot of weight to the Bollinger bands in these setups?
It's certainly possible! .6055 I'd like to see this puppy pinch.
SEEL daily
Under the $ for a minute there.
Wow. No wonder why I deleted this puppy.
Newer one today...
I guess I'll just wait till it goes under a buck to take a shot..........
Is it worth it at this point to start a position?
TIA
I’ve never owned a stock with 2 Reverse Splits.
SELL Seelos Therapeutics, Inc. Announces $4.0 Million Registered Direct Offering and Concurrent Private Placement Priced At-the-Market Under Nasdaq Rules
https://www.prnewswire.com/news-releases/seelos-therapeutics-inc-announces-4-0-million-registered-direct-offering-and-concurrent-private-placement-priced-at-the-market-under-nasdaq-rules-302045669.html
|
Followers
|
244
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
30393
|
|
Created
|
09/14/05
|
Type
|
Free
|
| Moderators | |||
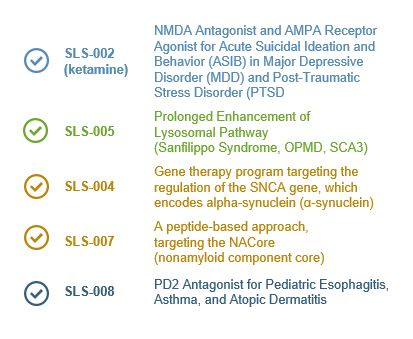
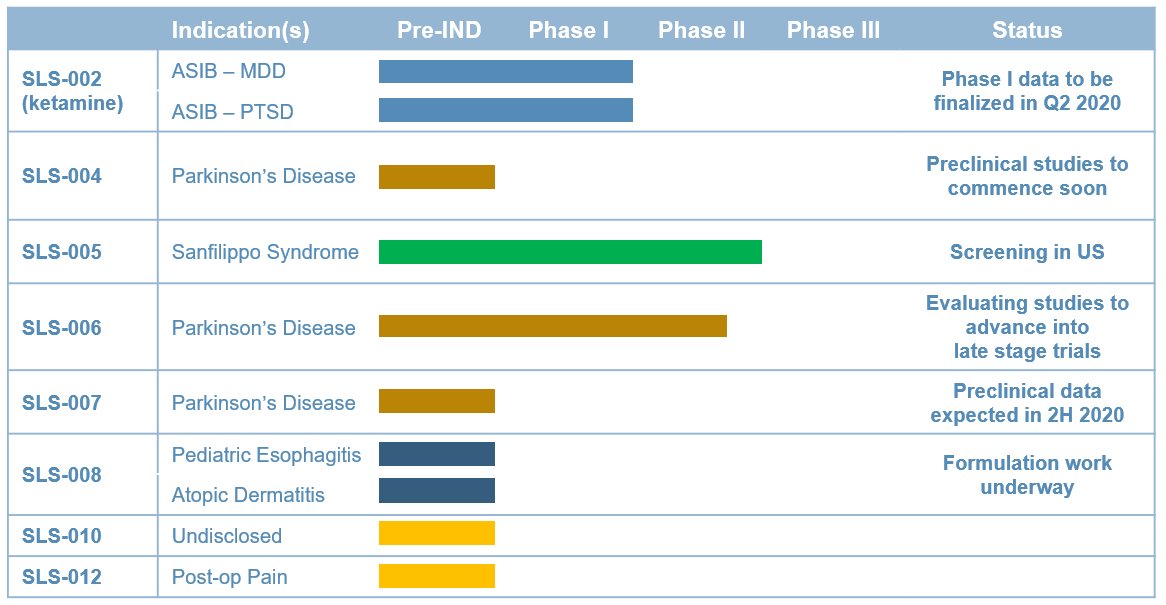
Seelos Therapeutics is exploring new ways to think about and treat central nervous system (CNS) disorders in neurological diseases and disorders and in rare diseases. Our trials are designed to further evaluate the safety and efficacy of our acquired assets. These come to Seelos with well characterized efficacy and safety, thereby avoiding much of the lengthy and burdensome process of preclinical R&D.
By working with clinically ready novel and reformulated compounds we will move from asset acquisition to meaningful data readouts in an expedited and efficient fashion. It is our goal to develop unique and disruptive therapies and approaches to improve treatment of large known diseases, offer new treatments for large unmet or recently characterized indications as well as advance therapies for orphan indications.
Our approach to this process will require development that is appropriate and efficient for a company our size in the scope of work each program requires in regards to trial size and duration.
We invite you to stay update on the progress of our assets in our clinical development programs.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
