Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Annovis Bio Receives FDA Approval to Transition to New Crystal Form of Buntanetap
MALVERN, Pa.
July 16, 2024 (GLOBE NEWSWIRE) -- via IBN – Annovis Bio Inc. (NYSE: ANVS) (“Annovis” or the “Company”), a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative disorders such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), announced today that it has received approval from the U.S. Food and Drug Administration (FDA) to transition to a new solid form of buntanetap in future clinical trials.
In June 2024, Annovis announced the filing of a composition of matter patent for the new crystal form of buntanetap and a provisional patent for the manufacturing process of this new form. The Company conducted comprehensive bridge studies in various solvents and in animals, comparing the old semi-crystalline form with the new crystalline form of buntanetap. Additionally, Annovis developed an innovative large-scale manufacturing process for the new form. This comprehensive data was submitted to the FDA for review.
The FDA has now approved the continuation of buntanetap's development using the new crystal form. This positive response allows Annovis to conduct a comparative study between the old and new forms of buntanetap in a small, single-dose, bioavailability study in humans as part of the transition process.
Agreed on the $20+ ANVS share price coming.
But if you want a Cancer Breakthrough treatment, take a look at RNAZ which has an extremely low OS if 6.6 million shares. They constantly get shorted same like ANVS does, but huge difference is the extremely low outstanding share count.
Hopefully we can see a cure for Parkinson’s, Alzheimer’s and cancer in short fashion. Good luck.
$ANVS look for continuation higher! 20.00+ coming.
Also loading breast cancer cure! $ICCM.
$ANVS
Isn't that the problem with almost every stock nowadays?
If it has to be,ANVS will succeed no matter the amount of shorting.
GLTY
The shorting is too strong. I'm concerned about getting back in.
Schwab's short sale Stock Borrow Rate is now at 729.75%
I don't know what to think about this anymore.
.
Maybe some have noticed the large 3 spikes up which stopped suddenly...Dec 2023, May of this year & July of this year...each high was progressively lower....the difference this time is the way the selloffs occurred....1st one was right away and continued for some days, the 2nd one was like a brick dropping from 5 stories up to a crash bottom...this one (so far) has not been as sharp or massive bust; it's early so I would think the trading might hold above the 200 day sma (9.34)...I'm watching, but certainly think Maria does her best to communicate with shareholders and has the boldness to file with the FDA and present her case for approval at this time!
https://bigcharts.marketwatch.com/advchart/frames/frames.asp?show=&insttype=&symb=anvs&x=0&y=0&time=8&startdate=1%2F4%2F1999&enddate=4%2F12%2F2018&freq=1&compidx=aaaaa%3A0&comptemptext=&comp=none&ma=1&maval=55550&uf=8&lf=2&lf2=8&lf3=4&type=4&style=350&size=4&timeFrameToggle=false&compareToToggle=false&indicatorsToggle=false&chartStyleToggle=false&state=11
Constant shorting here is amazing. It’s like the Wild Wild West OTC in here the way they short it dramatically only to score and then see the huge rises again.
Good for you you Xena!
Guess he is human after all
Didn’t think the head Dr Fauci
was Valdemort, Bad Trump.
Trumps not God, he didn’t believe
the EVIL that so many have chosen.
Don’t blame Trump, blame those that choose evil over humanity
Interesting comment, but as far as Trump is concerned --- the COVID scam started during his term and he did nothing to stop it.
I have no faith in him either.
Anavex doesn’t and won’t do anything for severe AD..
Not enough S1r cells to activate
Annovis IMO clears the channels for improvement but doesn’t necessarily stop
Why Dementia started..
I think they would make a great COMBO
Start with Annovis to Clear the Webs
and then engage anavex as a constant web sweeper..
But alas, everyone knows more than me.
Everyone is so much smarter than little old me.. I saw this 5 years ago and reached ou t to anavex but Chris didn’t have the time to talk to Maria.. so there you go..
Two safe drugs that AD patients should have already had.. surprised they don’t give both to Dementia Joe!
I don’t plan on meeding either anymore
with my X39 button feom LifeWave.com.
And until Trump destroys the beast that our FDA, FBI, NHS DOJ FBI have become
none of this really matters..
It’s all kabuki theater
So checking out Seeking Alpha today....
[Three things to note here:
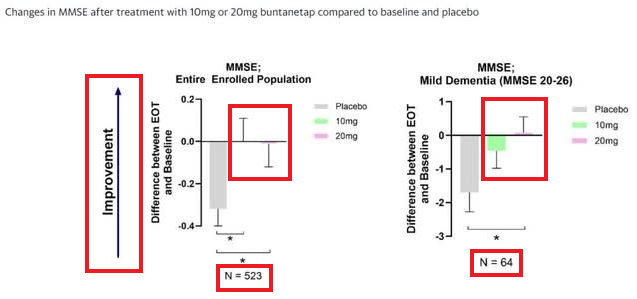
The "dose-dependent and statistically significant improvements in cognition in the overall enrolled PD population" (n=523) is an inverse one, i.e., 10mg improved more than 20mg.
This inverse "dose-dependent" improvement in cognition occurs in the overall enrolled patients (n=523), but is reversed in the "Mild Dementia MMSE 20-26" subgroup, where the 10mg group has no statistically significant improvement.
MMSE improvement data is the only data reported from the entire enrolled patients (n=523) in the PR./quote]
https://seekingalpha.com/article/4702593-annovis-bio-a-close-look-at-their-parkinsons-trial-data
I've been following Anavex for years, and I believe their approach is better,
Anavex Life Sciences Reports New Mechanism of Action Data Related to ANAVEX Compounds Targeting Sigma-1 Receptor
?
?
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental diseases including Alzheimer’s disease, other central nervous system (CNS) diseases, pain and various types of cancer, announced today the presentation of new mechanism of action data related to ANAVEX compounds targeting the sigma-1 receptor at the AD/PDTM 2017 Meeting.
Hall H et al presented preclinical data indicating that during pathological conditions, ANAVEX 3-71 demonstrated the formation of new synapses between neurons (synaptogenesis) without causing an abnormal increase in the number of astrocytes. In neurodegenerative diseases like Alzheimer’s and Parkinson’s disease, synaptogenesis is believed to be impaired.
Goguadze N et al presented preclinical data indicating that in addition to reducing oxidative stress, ANAVEX 2-73, ANAVEX 3-71 and ANAVEX 1-41 also demonstrated protective effects of the mitochondrial enzyme complexes I and IV during pathological conditions. The mitochondrial enzyme complex IV is directly involved in the synthesis of ATP, which provides energy within cells for metabolism. It is believed that energy production impairment and mitochondrial dysfunction play a role in the pathogenesis of neurodegenerative disorders and neurodevelopmental diseases.
“These new results provide converging evidence on mechanism of action as well as possible disease-modifying properties potentially by restoring homeostasis by means of the sigma-1 receptor agonists in our pipeline. This data also provides a foundation for our ongoing translational research efforts,” said Christopher U. Missling, PhD, President and Chief Executive Officer of Anavex.
Both data sets described that Aß1-42 (amyloid beta) significantly impairs biological function in the respective animal models and the observed regain of function confirmed the protective potential of ANAVEX sigma-1 receptor agonists against amyloid beta -induced toxicity. The former presentation further reported that although that the transgenic McGill rats had a very late stage of Alzheimer’s disease-like pathology (13 months of age), treatment with ANAVEX 3-71 fully reversed cognitive deficits, reduced amyloid pathology and also significantly reduced inflammation. Furthermore, the sustained recovery in cognition and pathophysiology observed following a month-long washout phase in advanced pathology (19 months of age) strongly suggests true disease-modifying properties of ANAVEX 3-71.
Search Labs | AI Overview
Learn more
…
No, clearing amyloid plaque alone is not likely to stop Alzheimer's disease. While amyloid plaque buildup is a hallmark of Alzheimer's disease, researchers believe that other factors also contribute to its progression, such as tau protein tangles, immune function, and vascular health. In fact, some research suggests that 25% of people with a clinical diagnosis of Alzheimer's disease have little to no amyloid in their brains.
The chart shows 5 minutes, apparently some trades were voided...

07/09/24 09:42:17 14.6342 14.55 14.85 505
07/09/24 09:42:16 14.60 14.55 14.90 1,406
07/09/24 09:42:15 14.7217 14.55 14.90 50
07/09/24 09:42:13 14.7217 14.55 14.90 25
07/09/24 09:42:13 14.7217 14.55 14.90 169
07/09/24 09:42:11 14.68 14.50 14.90 1,111
07/09/24 09:42:09 14.835 14.77 14.90 200
07/09/24 09:42:08 14.8947 14.77 14.90 25
07/09/24 09:42:08 14.8947 14.77 14.90 1,111
07/09/24 09:42:06 14.78 14.77 14.78 101
07/09/24 09:42:05 14.78 14.77 14.90 2,848
07/09/24 09:42:03 14.79 14.77 14.90 1,941
07/09/24 09:42:02 14.7788 14.77 14.78 213
07/09/24 09:42:01 14.77 14.77 14.78 105
07/09/24 09:42:00 14.77 14.77 14.78 1,123
07/09/24 09:41:59 14.77 14.77 14.78 2,425
07/09/24 09:41:58 14.835 14.77 14.90 538
07/09/24 09:41:57 14.77 14.77 14.90 4,220
07/09/24 09:41:55 14.999 14.93 15.00 25
07/09/24 09:41:53 14.999 14.93 15.00 400
07/09/24 09:41:52 14.9999 14.93 15.00 50
07/09/24 09:41:52 14.9999 14.93 15.00 4,504
07/09/24 09:41:51 14.965 14.93 15.00 1,359
07/09/24 09:41:50 14.97 14.93 15.00 1,411
07/09/24 09:41:48 14.9427 14.93 15.00 2,766
07/09/24 09:41:47 14.99 14.93 15.00 301
07/09/24 09:41:46 14.98 14.85 15.00 150
07/09/24 09:41:45 15.00 14.85 15.00 3
07/09/24 09:41:45 15.00 14.85 15.00 1,389
07/09/24 09:41:43 14.91 14.85 14.99 20
07/09/24 09:41:40 14.91 14.85 14.97 50
07/09/24 09:41:40 14.91 14.85 14.97 50
07/09/24 09:41:40 14.91 14.85 14.97 1,551
07/09/24 09:41:39 14.97 14.85 14.97 535
07/09/24 09:41:37 14.96 14.77 14.97 600
07/09/24 09:41:36 14.95 14.77 14.97 1,250
07/09/24 09:41:36 14.7701 14.77 14.97 399
07/09/24 09:41:34 14.7801 14.77 14.97 968
07/09/24 09:41:33 14.95 14.77 14.97 1,000
07/09/24 09:41:33 14.94 14.77 14.97 609
07/09/24 09:41:31 14.96 14.77 14.97 205
07/09/24 09:41:30 14.875 14.77 14.97 1,476
07/09/24 09:41:29 14.77 14.77 14.98 202
07/09/24 09:41:28 14.97 14.77 14.98 5,927
07/09/24 09:41:27 14.76 14.51 14.77 1,400
07/09/24 09:41:26 14.70 14.51 14.77 155
07/09/24 09:41:25 14.7037 14.51 14.77 1,309
07/09/24 09:41:24 14.666 14.51 14.77 300
07/09/24 09:41:23 14.71 14.51 14.77 1,049
07/09/24 09:41:22 14.60 14.50 14.77 100
07/09/24 09:41:21 14.62 14.50 14.77 63
07/09/24 09:41:19 14.62 14.47 14.77 1,562
07/09/24 09:41:19 14.60 14.47 14.77 678
07/09/24 09:41:18 14.62 14.47 14.77 200
07/09/24 09:41:17 14.66 14.47 14.77 963
07/09/24 09:41:16 14.65 14.47 14.70 1,637
07/09/24 09:41:14 14.55 14.47 14.65 2
07/09/24 09:41:14 14.55 14.47 14.65 4,772
07/09/24 09:41:13 14.58 14.45 14.65 181
07/09/24 09:41:12 14.513 14.45 14.65 3,101
07/09/24 09:41:11 14.435 14.40 14.41 135
07/09/24 09:41:10 14.435 14.40 14.47 570
07/09/24 09:41:09 14.435 14.40 14.47 4,895
07/09/24 09:41:08 14.435 14.40 14.47 546
07/09/24 09:41:06 14.47 14.40 14.47 371
07/09/24 09:41:06 14.435 14.40 14.47 1,172
07/09/24 09:41:05 14.41 14.40 14.47 137
07/09/24 09:41:04 14.4683 14.40 14.47 485
07/09/24 09:41:02 14.43 14.40 14.47 100
07/09/24 09:41:02 14.28 14.27 14.47 206
07/09/24 09:41:01 14.3337 14.27 14.40 2
07/09/24 09:41:00 14.3337 14.27 14.40 1
07/09/24 09:40:58 14.3337 14.27 14.40 101
07/09/24 09:40:56 14.3999 14.27 14.40 25
07/09/24 09:40:56 14.3999 14.27 14.40 2,622
07/09/24 09:40:55 14.40 14.27 14.40 104
07/09/24 09:40:55 14.2701 14.27 14.40 650
07/09/24 09:40:53 14.30 14.20 14.40 76
07/09/24 09:40:51 14.30 14.20 14.40 37
07/09/24 09:40:50 14.30 14.20 14.40 154
07/09/24 09:40:50 14.30 14.20 14.40 410
07/09/24 09:40:48 14.295 14.20 14.40 21
07/09/24 09:40:48 14.295 14.20 14.40 1,994
07/09/24 09:40:46 14.3278 14.29 14.40 126
07/09/24 09:40:43 14.3278 14.29 14.40 20
07/09/24 09:40:43 14.3278 14.29 14.40 18
07/09/24 09:40:43 14.3278 14.29 14.40 355
07/09/24 09:40:42 14.35 14.29 14.40 39
07/09/24 09:40:40 14.35 14.29 14.40 110
07/09/24 09:40:40 14.2001 14.29 14.40 1,200
07/09/24 09:40:39 14.25 14.20 14.30 45
07/09/24 09:40:37 14.25 14.20 14.30 620
07/09/24 09:40:37 14.31 14.20 14.30 3,651
07/09/24 09:40:35 14.385 14.30 14.47 412
07/09/24 09:40:34 14.40 14.28 14.47 129
07/09/24 09:40:32 14.45 14.28 14.47 16
07/09/24 09:40:32 14.45 14.28 14.47 50
07/09/24 09:40:31 14.45 14.28 14.47 31
07/09/24 09:40:30 14.45 14.28 14.47 354
07/09/24 09:40:29 14.30 14.21 14.47 3,700
07/09/24 09:40:27 14.289 14.20 14.29 1,200
07/09/24 09:40:26 14.24 14.20 14.29 243
07/09/24 09:40:25 14.20 14.19 14.29 90
07/09/24 09:40:24 14.20 14.19 14.29 816
07/09/24 09:40:22 14.15 14.10 14.20 401
07/09/24 09:40:22 14.199 14.10 14.20 2,491
07/09/24 09:40:21 14.12 14.10 14.20 25
07/09/24 09:40:20 14.12 14.02 14.20 1,134
07/09/24 09:40:19 14.1999 14.02 14.20 4,300
07/09/24 09:40:17 14.11 14.02 14.20 674
07/09/24 09:40:17 14.13 14.09 14.20 151
07/09/24 09:40:16 14.145 14.09 14.20 106
07/09/24 09:40:14 14.17 14.09 14.20 10
07/09/24 09:40:14 14.17 14.09 14.20 700
07/09/24 09:40:13 14.09 14.09 14.20 610
07/09/24 09:40:10 14.155 14.02 14.20 1
07/09/24 09:40:10 14.155 14.02 14.20 1,070
07/09/24 09:40:08 14.15 14.02 14.29 191
07/09/24 09:40:08 14.1352 14.02 14.29 2,718
07/09/24 09:40:07 14.19 14.10 14.29 2,194
07/09/24 09:40:05 14.155 14.02 14.29 725
07/09/24 09:40:01 14.155 14.02 14.29 20
07/09/24 09:40:01 14.155 14.02 14.29 3
07/09/24 09:40:01 14.155 14.02 14.29 71
07/09/24 09:40:01 14.155 14.02 14.29 586
07/09/24 09:40:01 14.02 14.02 14.29 935
07/09/24 09:40:00 14.281 14.19 14.29 951
07/09/24 09:39:59 14.29 14.19 14.29 1,538
07/09/24 09:39:57 14.17 14.19 14.29 616
07/09/24 09:39:56 14.105 14.02 14.19 100
07/09/24 09:39:56 14.0217 14.02 14.19 1,052
07/09/24 09:39:55 14.105 14.02 14.19 991
07/09/24 09:39:53 14.105 14.02 14.19 301
07/09/24 09:39:53 14.07 14.02 14.19 1,473
07/09/24 09:39:51 14.145 14.10 14.19 216
07/09/24 09:39:51 14.15 14.10 14.19 6,350
07/09/24 09:39:50 13.89 13.87 13.90 1,109
07/09/24 09:39:48 13.87 13.85 13.89 1,521
07/09/24 09:39:47 13.82 13.75 13.89 3,495
07/09/24 09:39:47 13.76 13.75 13.85 1,550
07/09/24 09:39:46 13.77 13.75 13.79 200
07/09/24 09:39:45 13.77 13.75 13.79 2,932
07/09/24 09:39:43 13.76 13.75 13.85 702
07/09/24 09:39:42 13.80 13.75 13.89 2,159
07/09/24 09:39:40 13.89 13.75 13.90 407
07/09/24 09:39:39 13.90 13.75 13.90 2,737
07/09/24 09:39:38 13.84 13.75 13.90 2,400
07/09/24 09:39:37 13.7901 13.75 13.80 329
07/09/24 09:39:36 13.81 13.75 13.90 40
07/09/24 09:39:35 13.81 13.75 13.90 332
07/09/24 09:39:35 13.80 13.75 13.90 3,335
07/09/24 09:39:33 13.7701 13.77 13.90 3,487
07/09/24 09:39:32 13.7501 13.75 13.76 266
07/09/24 09:39:30 13.7501 13.75 13.76 10
07/09/24 09:39:30 13.7501 13.75 13.76 446
07/09/24 09:39:29 13.76 13.75 13.90 1,312
07/09/24 09:39:28 13.7935 13.75 13.90 16,889
07/09/24 09:34:10 13.95 13.52 14.85 125
07/09/24 09:34:08 13.95 13.94 13.95 102
07/09/24 09:34:07 13.95 13.94 13.95 10
07/09/24 09:34:06 13.95 13.94 13.95 52
07/09/24 09:34:05 13.95 13.94 13.95 101
07/09/24 09:34:03 13.95 13.94 13.95 501
07/09/24 09:34:03 13.95 13.94 13.95 25
07/09/24 09:34:02 13.95 13.92 13.95 191
07/09/24 09:34:01 13.95 13.92 13.95 137
07/09/24 09:34:00 13.95 13.92 13.95 100
07/09/24 09:33:59 13.95 13.92 13.95 200
07/09/24 09:33:58 13.95 13.92 13.95 1,730
07/09/24 09:33:56 13.95 13.92 13.95 218
07/09/24 09:33:55 13.96 13.92 13.95 25
07/09/24 09:33:54 13.96 13.92 14.28 61
07/09/24 09:33:54 13.96 13.92 14.28 2,784
07/09/24 09:33:51 13.96 13.92 14.29 160
07/09/24 09:33:51 13.96 13.92 14.29 2,427
07/09/24 09:33:51 13.95 13.92 14.00 4,716
07/09/24 09:33:49 13.95 13.92 13.95 3,217
07/09/24 09:33:48 13.95 13.92 13.95 16,076
07/09/24 09:33:47 14.50 14.49 14.81 200
07/09/24 09:33:46 14.4901 14.49 14.81 4,481
07/09/24 09:33:45 14.64 14.49 14.80 5,105
07/09/24 09:33:44 14.66 14.52 14.81 25,949
07/09/24 09:33:42 15.15 15.09 15.24 600
07/09/24 09:33:41 15.09 15.15 15.24 109
07/09/24 09:33:41 15.09 15.09 15.24 115
Did you see what they tried to do this morning?
Got to $16.00 -- massive dump down to $13.94 -- trading halt -- now trading at $17.00
No shares available to short -- not even 100 shares.
This is looking more and more like a squeeze in the future.
Good to know!!
ANVS
Those are shorts with "connections"....
Tried to short 1,000 shares on this. They're saying there's no more shares available to short this. But all I see today are shorts trying to bring this down.
ANVS between 1-2 billion buyout, sometime this year.
LOL -- around 3:00 EST, this hit $16.50 -- and then dropped back to $15.00 in a matter of seconds. Shorting on this stock is restricted...there's no shares available to short. I think we're about to see a squeeze on this.
Always loved this ticker. Money is in the big board stocks. Holding only a couple of OTC's. Icahn and Jones.
It looks good !!
I’m going to buy some.
Tried to short 1,000 shares on this. They're saying there's no more shares available to short this. But all I see today are shorts trying to bring this down.
$12.83 Broke through $12.93 and $13.89. Accumulation and will break a second time then $18.01 in sight again
Stocks that will disrupt PD AD $ANVS
Oncology non surgical Freezing indication destroying malignant cancers $ICCM
My vertical call spreads in ANVS are up 754% from a month ago. Will sell my calls tomorrow take initial investment out buy and ride shares until Buyout or FDA approval or what ever the future holds for ANVS very hard market to prove data & move the needle in a significant way in PD and AD. I believe other investors in this area will sell other positions and build a starter in Annovis Bio in the coming days and weeks.
Annovis Pipeline
https://www.annovisbio.com/our-science
Annovis Bio Announces New Data from Phase III Parkinson’s Study Highlighting Improvements in Unified Parkinson's Disease Rating Scale (MDS-UPDRS) and Cognition after Treatment with Buntanetap
https://irpages2.eqs.com/websites/annovis/English/431010/us-press-release.html?airportNewsID=87ac07b2-1a3d-49b7-af9e-ab0ecdfd5310
$ANVS
ANVS Sold 2/3 in AH @ 11.08 for a +7.6% win
Let's see what Monday brings.
Just missed an 11.38 exit in regular session by 3 cents.
I agree. I'm waiting to see what happens before I jump back in.
Seems like the shorts are getting nervous with todays sudden ASSSSSSSK Slapping!
Trump gonna change it ALL
Someone is selling...which entices short selling. But someone is also buying. And the buying has led me to hold and see where this goes.
Last week
Seeking Alpha allowed me to read
a post regarding what a
Great Short Annovis is..
What sickness lies at the hearts of men?
Why would you short a company trying to save suffering human beings?
We need a new path for non revenue bio pharmaceutical companies and it doesn’t run through our current Fed bureaucracies
Now
Make the 20 mg available now
They allow experimental Covid Jabs that cause myocarditis to unknowledgeavle healthy children! Let knowledgeable death sentenced adults make a choice about this!
God Bless You Maria and your entire team!
ANVS: Indeed!! (And 5% up more, right now, in the Post-M too. At my first review of their news, I thought it was about Alzheimer's affliction, which has a much larger USA patients base vs. Parkinson's!!)
After hours looks strong. I hope tomorrow morning is as strong as well.
ANVS $10.30 Still has room $18.01- $19.85 are the longer term resistance levels. See how it rolls
Seems 50 is easier and 500m cap only.
|
Followers
|
45
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
424
|
|
Created
|
02/04/21
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
