Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
AKTX.................................https://stockcharts.com/h-sc/ui?s=AKTX&p=W&b=5&g=0&id=p86431144783
AKTX.........................................https://stockcharts.com/h-sc/ui?s=AKTX&p=W&b=5&g=0&id=p86431144783
AKTX.......................................https://stockcharts.com/h-sc/ui?s=AXTX&p=W&b=5&g=0&id=p86431144783
AKTX..................................https://stockcharts.com/h-sc/ui?s=AKTX&p=W&b=5&g=0&id=p86431144783
Akari Therapeutcis (AKTX) Presesnts Positive Results from Two Preclinical Development Programs of Long-Acting PAS-Nomacopan
Akari Therapeutics, plc (Nasdaq: AKTX) presented positive results from two preclinical studies of its lead asset, investigational nomacopan, in diseases of the eye at the Association of Research in Vision and Ophthalmology (ARVO) 2022 Annual Meeting. The two presentations are available at www.investor.akaritx.com/news-and-events/presentations.
“There is significant unmet need in ophthalmology, and we are encouraged by the results of our work so far in the development of long-acting PAS-nomacopan for geographic atrophy,” said Rachelle Jacques, President and CEO of Akari Therapeutics. “This will be an area of focus and investment for Akari as we drive this program forward.”
Development of long-acting PASylated-nomacopan for treatment of GA and other retinal diseases
Geographic atrophy (GA) is a chronic progressive degeneration of the macula, which occurs during late-stage dry age-related macular degeneration (dAMD). Over time, GA can lead to permanent vision loss. It is estimated that more than 8 million people worldwide are affected by GA in AMD and currently there are no approved treatments.
Studies have indicated that while certain complement inhibitors slow the progression of GA, they may also promote choroidal neovascularisation (CNV), which can harm the macula, damage vision,1,2 and require VEGF rescue therapy.
Leukotriene B4 (LTB4) is a potent leukotactic agent that can increase retinal vascular endothelial growth factor (VEGF) a key driver of CNV. Inhibition of LTB4 may decrease the risk of CNV.3 Akari has conducted pre-clinical studies that explore the importance of the LTB4-VEGF axis and the potential role of nomacopan’s bispecific inhibition of both C5 and LTB4 in treating GA/dAMD. In a non-infectious allergic uveitis animal model, PAS-nomacopan reduced VEGF by more than 50% compared to saline control, equivalent to the inhibition caused by an anti-VEGF antibody. In addition, PAS-nomacopan was significantly more effective in reducing retinal inflammation than the anti-VEGF antibody.
One of the pre-clinical studies presented at ARVO 2022 used an industry standard model of laser-induced CNV. Intravitreal (IVT) PAS-nomacopan injected once during a 16-day treatment period was compared to a U.S. Food and Drug Administration-approved VEGF inhibitor for impact on neo-vascularization. The IVT single dose of PAS-nomacopan significantly reduced CNV (p=0.022) as compared to saline and was as effective as multiple IVT injections of the VEGF inhibitor (p=0.019.) Single IVT injection of PAS-nomacopan showed a trend towards reduced leakage on Day 14 (p = 0.097).
Currently approved therapies for retinal diseases injected directly into the vitreous cavity are typically administered monthly. Studies have shown that due to adverse effects (such as an increase in intraocular pressure [IOP]), discomfort and anxiety, IVT injection presents a heavy burden on patients. PASylation of nomacopan has the potential to make it long-lasting in the back of the eye and may provide a dosing interval that is more attractive to patients.
Akari is continuing PK and PD work to optimize PAS-nomacopan with the aim of achieving safety and efficacy in GA, and meeting patient preferences for less frequent injections
Comparison of topical nomacopan, a dual complement and LTB4 inhibitor with dexamethasone in downregulating experimental immune-mediated conjunctival disease (EIC)
Steroid-resistant allergic conjunctivitis, including atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC), is difficult to treat and can lead to corneal scarring and vision loss. Topical or systemic dexamethasone and/or cyclosporin A (CsA) is often required, however dexamethasone may be associated with adverse reactions, including increased IOP.
Topical administration of nomacopan, a therapeutic protein, was recently shown to be effective at attenuating inflammation in a model of experimental immune-mediated conjunctivitis (EIC). The aim of the study presented at ARVO 2022 was to compare the anti-inflammatory effects of nomacopan with topical dexamethasone.
IL-9 expressing mast cells and CD4+T cells are upregulated during ovalbumin (OVA)-induced EIC.
In the preclinical study presented at ARVO 2022, nomacopan preferentially downregulated conjunctival Th2 IL9 producing, Th2 and Th9 CD4+T-cells and nomacopan, dexamethasone and cyclosporinA all effectively decreased Th2 and Th9 cells in draining lymphnodes (dLNs). These findings support use of topical nomacopan to treat allergic eye diseases including VKC and AKC.
References:
Liao DS, et al. Ophthalmology. 2020 Feb;127(2)
Jaffe GJ et al. Ophthalmology. 2021 Apr;128(4)
Sasaki F et al. JCI Insight. 2018 Sep 20;3(18)
Completely doomed company. Nobody touching it! More vol pre-market than trading.
Funny to read the messages from 2 years ago. Same price, sme story. Deals in the making, building in silence. Partnership coming soon. Yeah 24 month later nothing happend. Do not waste your money, this is a dead horse!
yeah, pretty awful.
Long term chart a mess. 4 pump and dumps with lower and lower spikes. now flatline. Everybody is so excited that the constant buying leads to falling prices. Every child knows something is not adding up.
Prices go down when people sell, people sell, when company is shit.
Akari Therapeutics P (AKTX)
1.68 ? 0.03 (1.82%)
Volume: 96,497 @08/27/20 3:35:34 PM EDT
Bid Ask Day's Range
1.67 1.68 1.62 - 1.69
AKTX Detailed Quote
mick, old pump and dumper. Now 10% lower. When is the pump starting or do we make 1000% from 20 cents?
$AKTX Akari Therapeutics P (AKTX)
1.89 ? -0.01 (-0.53%)
Volume: 318,481 @08/07/20 7:53:46 PM EDT
Bid Ask Day's Range
1.86 1.9 1.85 - 1.94
AKTX Detailed Quote
$AKTX BIG TIME MOVE
Added a bunch Friday $AKTX. Have bigly position now. Will wait for double digits to sell. Likely news next week prior to the ER imo
$AKTX Akari Therapeutics PLC (NASDAQ: AKTX): interim update on the Part B placebo-controlled efficacy arm of the study of nomacopan eye drops in atopic keratoconjunctivitis
$AKTX going to be huge runner my friend !!!!!
re;
I was buying $capr at 90¢ and been predicting run like this for weeks. $AKTX will do same or better imo.
Has history of monster runs.
Insiders like chairman own like 50% of the company.
7th richest man in japan holds like 15% of the co.
A doctor from Texas owns 10%+ of the company. OS is 30M.
What size publicly tradable float is left?
Hmmmmmmm $AKTX
ans
thank you
$AKTX i am looking fer $8 plus !!!!!
$AKTX why not this wk ????? biotech are hot item !!!!!
re;
Excellent to see some new posters here.
Give this stock a few months and you will see have a nice movement.
They got the same funding that v k t x recieved.
I saw them move from $1.43 to $23.
I am hoping we have a similar move here at some point.
The science here is very good.
I met the CEO once when they presented in Vancouver.
He is very much a straight shooter.
The majority of the faculty here are Oxford graduates.
All good fellows, with the intention of getting things done right.
ans.
did $AKTX receive funding ????? PPP ?????
$AKTX Akari Therapeutics P (AKTX)
1.97 ? -0.1 (-4.83%)
Volume: 273,514 @07/29/20 6:57:09 PM EDT
Bid Ask Day's Range
1.96 2.02 1.95 - 2.0491
AKTX Detailed Quote
$AKTX I AGREE WITH PPS , EXCELLENT
Agreed. And I'm watching $AKTX for signs to buy more.
Folks don't seem to respond quickly to Biotechs, the
way we do with the more 'sexy stocks'. Bios usually need
hard evidence / data, to get buyer reactions, IMHO.
GLTA
Excellent to see some new posters here. Give this stock a few months and you will see have a nice movement. They got the same funding that v k t x recieved. I saw them move from $1.43 to $23. I am hoping we have a similar move here at some point. The science here is very good. I met the CEO once when they presented in Vancouver. He is very much a straight shooter. The majority of the faculty here are Oxford graduates. All good fellows, with the intention of getting things done right.
$AKTX here you have Akari’s medical director as a presenter for upcoming Covid conference. Obviously he knows he’d have some solid data to present or else they’d have not signed up Akari imo https://complement-therapeutics.com/speaker/wynne-weston-davis/
Meanwhile mkt is clueless Akari even has a Covid program. ?? https://twitter.com/rogerthat191/status/1287970088696463361/photo/1
I was buying $capr at 90¢ and been predicting run like this for weeks. $AKTX will do same or better imo. Has history of monster runs. Insiders like chairman own like 50% of the company. 7th richest man in japan holds like 15% of the co. A doctor from Texas owns 10%+ of the company. OS is 30M. What size publicly tradable float is left? Hmmmmmmm $AKTX
$AKTX YOU ARE WELCOME. AKTX Bid: 2.08 Ask: 2.11 Last: 2.11 Chg ($): 0.16 Vol: 386,725
Thanks for the post Mick. We are gold with $AKTX.
$AKTX Akari Therapeutics (AKTX) $5 target !!!!!
Last up to bat we have Akari Therapeutics, which develops innovative treatments for autoinflammatory diseases involving the complement (C5) and leukotriene (LTB4) pathways. Given its strong execution and share price of only $2.14, is now the right time to get in on the action?
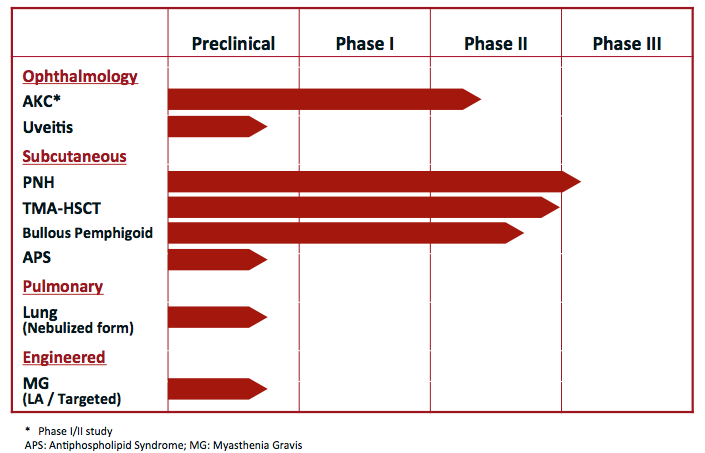
According to B.Riley FBR’s Mayank Mamtani, the answer is a resounding yes. At the end of May, the company provided an update on the progress of the clinical trials for bullous pemphigoid (BP), atopic keratoconjunctivitis (AKC) and pediatric transplant-induced thrombotic microangiopathy (HSCT-TMA) indications for its lead program, nomacopan. The candidate is also getting attention for its potential as a therapeutic option for COVID-19 patients.
The 5-star analyst thinks the full Phase 2 BP study results, as well as an orphan designation granted by the FDA and EMA, free AKTX up to kick off end-of-Phase 2 meetings with the FDA and EMA in Q3 2020 to discuss the pivotal Phase 3 trial design. These meetings would address important considerations including enrolling severe patients in addition to mild and moderate, increasing treatment duration from 1.5 to 6 months, an option to dose at 30 mg-plus levels and a choice of active versus comparator arms, including possibly combining or sequencing with steroids.
“There were no treatment-related serious AEs, in line with the favorable tolerability profile noted in other indications (e.g., PNH), highlighting further differentiation relative to steroid standard of care,” Mamtani stated.
However, Mamtani doesn’t dispute the fact that the COVID-19 pandemic has delayed site initiation activities for the Phase 3 HSCT-TMA trial to later in 2020 and the enrollment for the Part B placebo-controlled efficacy cohort of the Phase 1/2 study in severe AKC patients has been halted. That said, Mamtani notes “the interim update remains on track for mid-2020 in ~2/3rd of the targeted 16 patients already enrolled in the study.” He added, “The prior open-label data of nomacopan eye drops demonstrated in three severe atopic keratoconjunctivitis (AKC) patients rapid overall improvement in composite clinical scores of symptoms and signs.”
On top of this, preclinical data has found complement (C5) and leukotriene (LTB-4) pathways may play a role in severe lung inflammation and microthrombi and organ damage associated with COVID-19. This creates an opportunity for nomacopan as it has been shown to produce a “profound and broad-acting anti-inflammatory effect.”
To wrap it all up, Mamtani commented, “We believe AKTX has held up well in the volatile macro environment as investors look to take advantage of the depressed stock levels for this late-stage biotech supported by robust clinical data generated in earlier-stage trials, with incremental Phase 1/2 severe AKC placebo-controlled data anticipated in mid-2020.”
Everything the company has going for it prompted Mamtani to stay with the bulls. Along with a Buy rating, he kept his $5 price target as is, bringing the upside potential to 134%. (To watch Mamtani’s track record, click here)
Turning now to the rest of the Street, it has been quiet when it comes to other analyst activity. Mamtani’s call was the only one issued recently.
$AKTX reading your theory
re;
Should AKTX push nomacopan to market, the potential here would be incredible. There are no approved treatments that are indicated for HSCT-TMA patients. However, the market surrounding the condition is a massive one.
In fact, experts expect that the HSCT-TMA market will grow to be worth well over $37 billion annually by the year 2025. That’s a big deal, especially when you consider that Akari Therapeutics is currently trading with a sub-$50 million market cap.
With no competition on the playing field, this relatively small biotech company could have a blockbuster on its hands, should the treatment be approved. So, keep your eyes peeled as this opportunity could be huge.
$AKTX READING YOUR DD, outstanding stuff !!!!!
re;
AKTX NEWS this morning positive preclinical trials and has a history of big jumps from $2 - $10 overnight. Check out the 1 year chart
https://www.stockscores.com/charts/charts/?ticker=aktx
$AKTX i didn't look @ a.h. finished up !!!!!
Bid Ask Day's Range
1.95 2.04 1.94 - 2.0
AKTX Detailed Quote
$AKTX, i will try fer more shares !!!!!
She ready to go for a run imo
$AKTX THANX FOR UPDATING. how ya like 5yr. chart ?????
re;
Last year had the same pattern. Did a small private placement June 28 and then 1 month from that date started cranking out news.
This year they did the private placement July 1. Expecting news flow in next week or week after that. I call this Rogers machine learning $AKTX
$AKTX GOOD REPORE'
RE;
Last year had the same pattern.
Did a small private placement June 28 and then 1 month from that date started cranking out news.
This year they did the private placement July 1.
Expecting news flow in next week or week after that.
I call this Rogers machine learning $AKTX
ANS
I AM LISTENING !!!!!
Last year had the same pattern. Did a small private placement June 28 and then 1 month from that date started cranking out news.
This year they did the private placement July 1. Expecting news flow in next week or week after that. I call this Rogers machine learning $AKTX
$AKTX FIVE YR.
[-chart]www.stockscores.com/chart.asp?TickerSymbol=AKTX&TimeRange=1825&Interval=w&Volume=1&ChartType=CandleStick&Stockscores=1&ChartWidth=1100&ChartHeight=480&LogScale=&Band=&avgType1=&movAvg1=&avgType2=&movAvg2=&Indicator1=None&Indicator2=None&Indicator3=None&Indicator4=None&endDate=&CompareWith=&entryPrice=&stopLossPrice=&candles=redgreen[/chart]
$AKTX, I THINK $7 TO $10 PPS MY FRIEND
I FOLLOW YOU
I WILL MARK YOU TOO.
RE;
I’ve posted a lot of $AKTX DD on Twitter.
Just follow this thread imo. Co trading at $2 right now. But won’t be for too long imo.
$AKTX this chart really intrigues me. This ticker has been trading in this channel for like 2 years and now been trying to break out on no news? Got to ask why. Chairman owns half the co and co has several value drivers imminent. Excited to see what next week brings us. pic.twitter.com/efI4zbFiEK
— Rogerthat1 (@rogerthat191) June 27, 2020
I’ve posted a lot of $AKTX DD on Twitter. Just follow this thread imo. Co trading at $2 right now. But won’t be for too long imo.
$AKTX this chart really intrigues me. This ticker has been trading in this channel for like 2 years and now been trying to break out on no news? Got to ask why. Chairman owns half the co and co has several value drivers imminent. Excited to see what next week brings us. pic.twitter.com/efI4zbFiEK
— Rogerthat1 (@rogerthat191) June 27, 2020
$AKTX Akari Therapeutics P (AKTX)
1.95 ? -0.05 (-2.50%)
Volume: 115,195 @07/24/20 7:23:49 PM EDT
Bid Ask Day's Range
1.95 2.04 1.94 - 2.0
AKTX Detailed Quote
$AKTX “We remain focused on our mission of addressing severe inflammatory diseases with significant unmet medical need.
We are pleased that our company is now in the process of planning or undertaking pivotal trials in both BP and HSCT-TMA as well as
progressing next clinical steps for studies in
atopic keratoconjunctivitis (AKC)
and COVID-19 pneumonia,”
said Clive Richardson, Chief Executive Officer of Akari Therapeutics.
Severe inflammatory diseases remain an area of high unmet need.
Akari continues to make progress driving its anti-inflammatory programs forward, including the recent announcement of positive topline results
from our Phase II BP study in May.
$AKTX ]fast track approval Open pivotal study in pediatric hematopoietic stem cell transplant-related thrombotic microangiopathy (HSCT-TMA) with orphan and fast track approval
Preparing for pivotal study in bullous pemphigoid (BP) following recent
completion of successful Phase II study
Positive EMA opinion on orphan designation for nomacopan for BP along with
Orphan Drug designation granted by FDA in BP
https://finance.yahoo.com/news/akari-therapeutics-reports-first-quarter-110010702.html
$AKTX 1.9700 -0.0300 -1.50% USD 11:20AM EDT 55.152k
|
Followers
|
13
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
555
|
|
Created
|
09/21/15
|
Type
|
Free
|
| Moderators | |||
Akari is focused on developing inhibitors of acute and chronic inflammation, specifically the complement system, and leukotriene system,
for the treatment of rare and orphan diseases with unmet need. We believe that blocking early mediators of inflammation
will prevent initiation and continual amplification of the processes that cause certain diseases.
Nomacopan is a recombinant small protein (16,740 Da) that acts on complement component-C5,
preventing release of C5a and formation of C5b–9 (also known as the membrane attack complex, or MAC),
and independently also inhibits leukotriene B4, or LTB4, activity, both elements that are co-located as part of the immune/inflammatory response.
Nomacopan is currently being clinically evaluated in four indications:
Akari believes that the dual action of nomacopan on both C5 and LTB4 may be beneficial in AKC, BP, and aHUS. Akari is also developing other tick derived proteins, including longer acting versions.
Nomacopan is a highly soluble and stable biological molecule, and independently binds to both C5 and LTB4. This novel dual binding enables targeting of two distinct and separate disease categories based on their underlying etiology.
July 1, 2020
July 1, 2020
June 1, 2020
May 1, 2020
March 31, 2020
Patients with COVID-19 pneumonia
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
