Replies to post #87412 on Avid Bioservices Inc (CDMO)
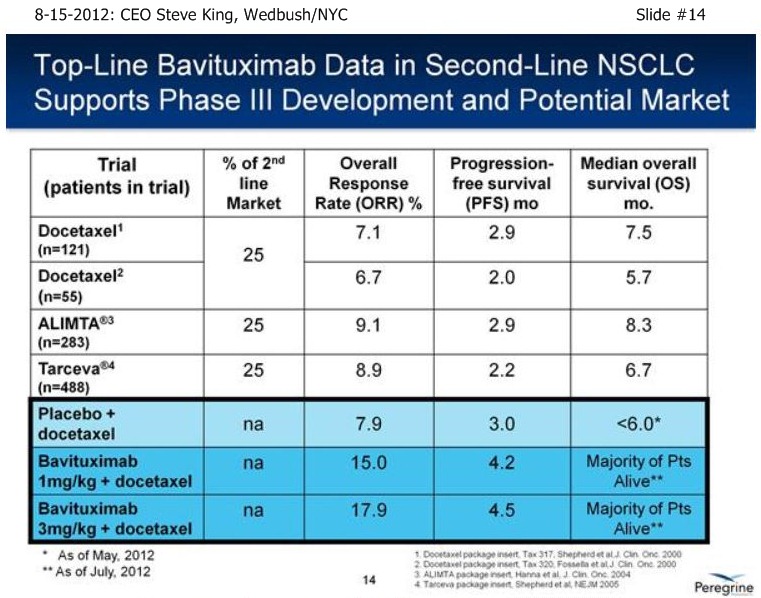
As we’re sitting here today, we have still not reached the # of events for MOS in either of the Bavituximab arms – and, in fact, we still have patients that are on treatments.





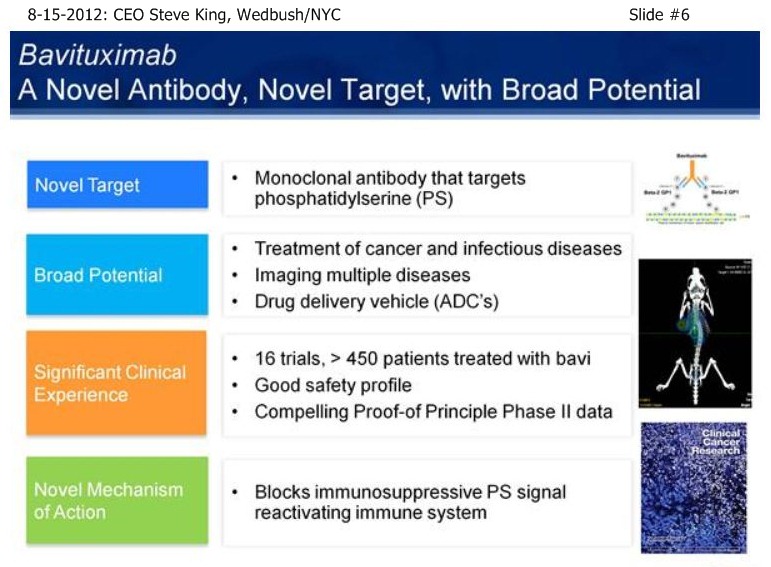
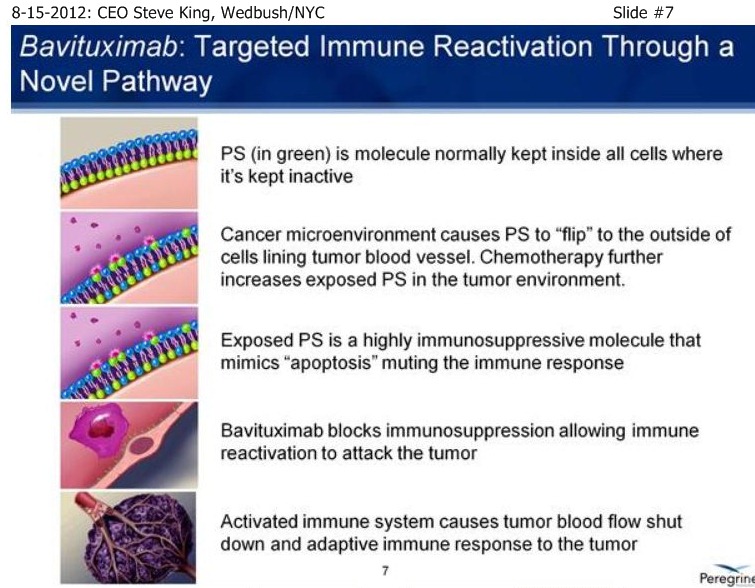
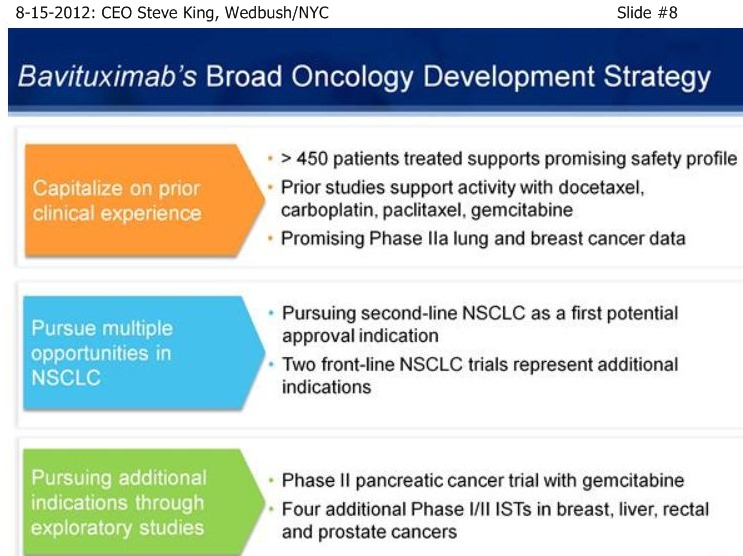

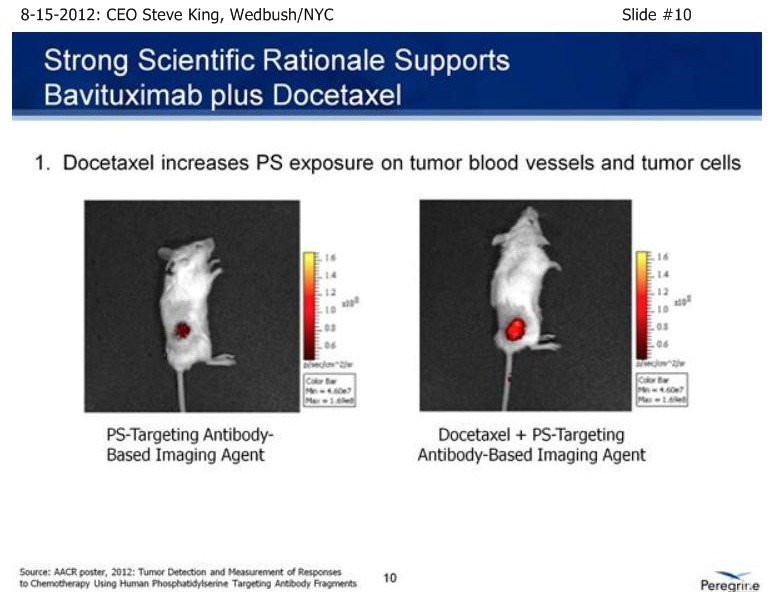
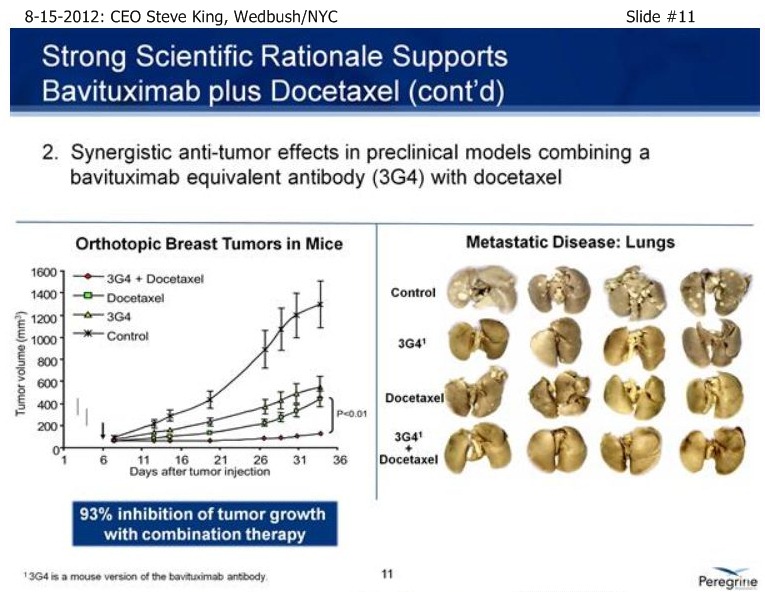

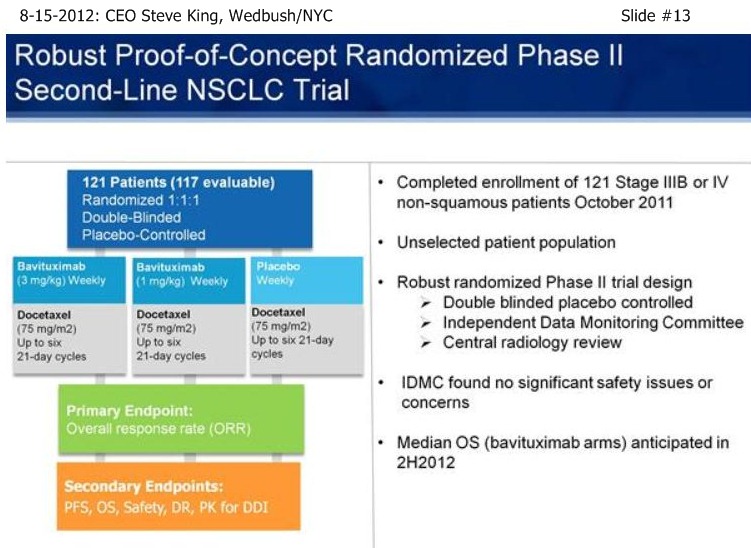



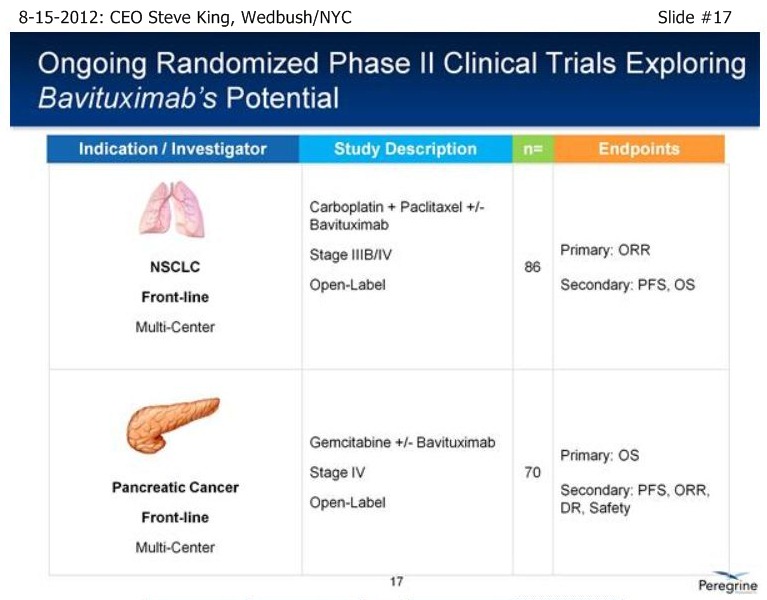
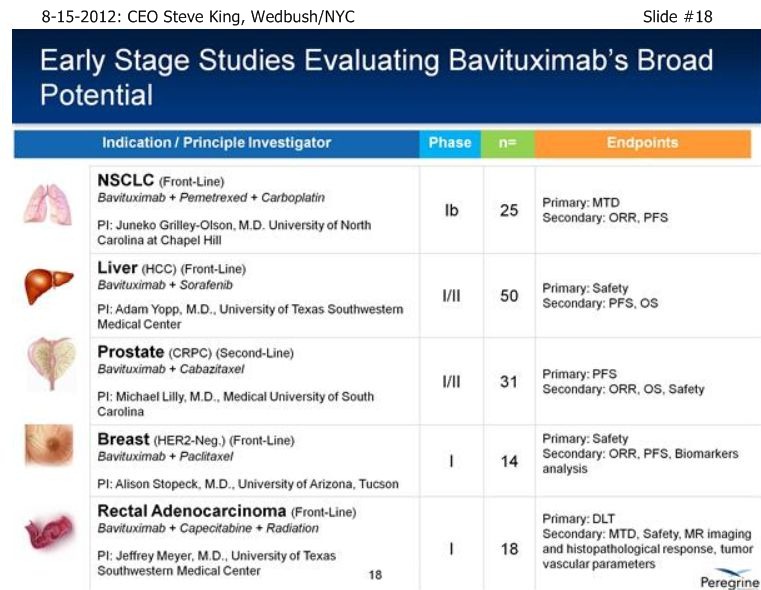



| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |