Tuesday, January 14, 2014 6:26:22 PM
START HERE. SVFC DD COMPILATION.
***Updated: 1/14/2014***
Table of Contents:
1) The Industry
2) The Company
3) The People
4) The Science
5) The Patents
6) The Due Diligence
1) The Industry
Regenerative Medicine (RM) is currently a $15 billion market and is expanding rapidly. In fact, many are calling this LARGELY UNTAPPED market "The Internet of Healthcare," and it is projected to reach $300 billion by year 2020 as patient advocacy groups demanding shift to RM proliferate worldwide (source: US Department of Health and Human Services). 1.2 million patients have been treated to date with RM products and therapies. Currently, 300 private and 50 public companies exist with $4.7 billion in total market capacity. $1.5 billion in worldwide research funding projected to $14 billion in 10 years.
2) The Company
Intellicell is a fully-reporting company currently trading on OTCQB.
The Company's Website
Synopsis:
Intellicell is an emerging leader in regenerative medicine using highly potent stromal vascular fraction cells derived from vasculature of fat tissue via uniquely efficacious technology that is patented in the United States with multiple patents pending internationally. The company is intentioned to enter the large and rapidly growing market of medical conditions where cellular therapies are far more effective than traditional techniques alone.
MOST RECENT SHARE STRUCTURE:
SHARES OUTSTANDING: 316,658,806 as of November 21, 2013 (per latest 10-Q)
AUTHORIZED SHARES: 1,500,000,000
3) The People
The CEO
Dr. Steven Victor, founder and CEO of IntelliCell has been at the forefront of clinical product and process development for over 20 years. The patent pending process that Dr. Victor has developed for IntelliCell™ has been in research for over 4 years. In addition to developing clinical products that are used nationally and internationally in the medical aesthetics field, Dr. Victor is a practicing dermatologist in New York City. He has been a sought after national figure for teaching physicians new clinical techniques worldwide for over 20 years. Dr. Victor has also been featured in national and local media as a clinical subject matter expert in regenerative medicine, medical aesthetics, and dermatology.
Watch the CEO talk about Intellicell below:
Board of Directors
Mr. Michael Hershman
IntelliCell BioSciences Appoints Mr. Michael Hershman as Chairman of the Board of Directors
Mr. Hershman is an internationally recognized expert on matters relating to transparency, accountability, governance, litigation and security. President and CEO of the Fairfax Group, a company he founded in 1983, that has been retained by governments, corporations, law firms and international financial institutions to assist on matters relating to the consult of senior-level officials and/or the entities with which they do business.
More on Mr. Hershman can be found on the website of Fairfax Group by following this link.
Here is a video in which Michael Hershman is awarded Columbia Business School’s 2013 Botwinick Prize in Business Ethics.
I suppose having a renowned business ethics expert and a successful businessman with multiple governmental connections as your BOD Chairman is not a bad thing ;)
Mr. Myron Holubiak
Myron Holubiak is currently President of 1-800-Doctors, Inc. Mr. Holubiak is also Chairman of the Board of Directors of BioScrip, Inc., an infusion and home health company, and Lead Independent Director of Ventrus BioSciences, Inc. From 1998 through 2001, Mr. Holubiak was President of Roche Laboratories, Inc. and previously in his career, he co-founded and served as CEO to Emron, Inc, a strategic marketing firm serving the pharmaceutical industry, which was acquired by Dunn & Bradstreet and IMS Health in 1995. Mr. Holubiak also currently serves on the Board of Trustees for the Academy of Managed Care Foundation.
Mr. Leonard L. Mazur
Mr. Leonard L. Mazur Co-founded Triax Pharmaceuticals, LLC and serves as its Chief Operating Officer. Mr. Mazur also Co-Founded Akrimax Pharmaceuticals in 2007. Mr. Mazur began his pharmaceutical career with Cooper Laboratories in 1971, where he served as a Product Manager for ophthalmology, dermatology and other products. He was responsible for creating and growing the business that was sold to Pierre Fabre, a French dermatology company, in 2002. In 1995, he founded Genesis Pharmaceutical, Inc., and served as its Chairman, Chief Executive Officer from 1995 to 2005 and President.
Mr. Sam Khashman
IntelliCell Biosciences Announces the Addition of Mr. Sam Khashman to Board of Directors
During the past 20 years, Mr. Khashman has successfully directed the creation and launch of 14 products and 3 companies in the healthcare, financial, and manufacturing sector. He currently serves as the President and Chief Executive Officer of Technology Partners, Inc. DBA IMAGINE Software. Mr. Khashman founded the company and created the IMAGINE practice management system that is currently used in 48 States by more than 10,000 physicians. In addition to his involvement in numerous civic and charitable organizations, Mr. Khashman serves on the board of the National Chamber Foundation, the public policy think tank of the U.S. Chamber of Commerce in Washington D.C.
Medical Advisory Board
Dr. James Andrews
IntelliCell BioSciences Inc. Announces James R. Andrews, M.D. Has Joined Its Medical Advisory Board
Doctor James Andrews is one of the founding members of Andrews Sports Medicine and Orthopaedic Center in Birmingham, Alabama. He is also a founder of the American Sports Medicine Institute (ASMI) a non-profit institute dedicated to injury prevention, education and research in orthopaedics and sports medicine. This foundation is recognized as one of the world’s leaders in this field. Doctor Andrews is internationally known and recognized for his skills as an orthopaedic surgeon as well as his scientific and clinic research contributions in knee, shoulder and elbow injury prevention and treatment. In addition, he has made major presentations on every continent, and has authored numerous scientific articles and books.
A list of athletic celebrities treated by Dr. Andrews as of 5 years ago can be found following this link.
Could some of his NEW celebrity patients have been treated with the help of Intellicell’s technology, but not yet publicized?
Dr. Joshua Hackel.
Another member of Andrews Institute on Intellicell’s board of medical advisors is Dr. Joshua Hackel. His profile information can be found here.
Dr. Sydney Coleman
You can read more about Dr. Coleman's extensive stem cell projects funded by the Department of Defense through the Office of the Assistant Secretary of Defense for Health Affairs in this post by lee13.
Note that these fantastic people are just part of the group currently working to move Intellicell’s technology forward. Complete corporate profile for the company can be found following this link. Just Google some of the names not mentioned in this post to see that Intellicell is associated with crème de la crème of medical and scientific communities.
4) The Science
According to this PR, an antibody flow cytometry study of IntelliCell's technology was performed by Millipore, a division of Merck (NYSE: MRK) and found that:
"IntelliCell's proprietary technology yield an average of 10 times (10X) the number of SVF cells containing adult adipose stem cells from less fat (only 2 oz) than that which the Company believes is used by any of its present competition utilizing enzymes in their process. The study also showed that IntelliCells™ contain all of the viable cells that are manufactured by competing technologies that use enzymatic digestion."
The following image presents cell culturing report using Intellicell’s technology:
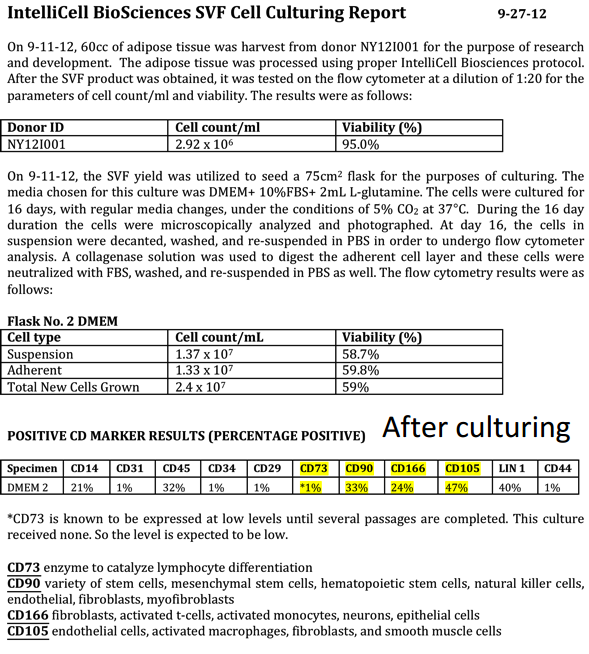
Moreover, it is because no enzymes are necessary in Intellicell’s process that they are the only public commercial stem cell company in the United States to meet FDA Rule 361 exemption. Under the FDA’s Rule 361, IntelliCells is currently authorized to commercially sell its SVF stem cells in the U.S. The PR announcing the most recent FDA inspection of the company’s flagship lab can be found here. The copy of the FDA’s report can be obtained at this link. Note that most of the observations cited in the report are minor and can be corrected easily. When the FDA spends four days in ANY lab, they WILL find something – it’s just the way the game goes.
Additionaly, the company previously announced that “it has received the results of its independent laboratory audit by Biologic Consultant Group and the audit showed that the new lab at 460 Park Avenue, New York, NY was cGTP compliant.”
Futhermore, as announced here, Intellicell “has been notified according to the FDA validation registration number 3009842420, that its new facility located at 460 Park Avenue, New York, NY 10022 is now registered to recover, process, package, store, and label human cells and tissue products (HCT/P's) such as the IntelliCell autologous stromal vascular fraction cellular product.”
Intellicell Biosciences wins key listing from the FDA
Finally, according to this news release, Intellicell “has been granted a Research Tissue Banking License by the New York State Department of Health.” Which will now allow the company to engage in “the development of autologous and allogenic stromal vascular fraction tissue for researchers around the world.”
A great summary of Intellicell’s technology as compared to current competitors can be found on page 19 of the company’s most recent investor presentation. The visual comparison is presented below:

A list of Intellicell’s current human clinical trials, as officially reported by the company in the latest investor presentation can be found in this post by lee13.
Here is an additional link to a prospective pilot study using Intellicell’s technology on the successful clinical application of SVFC on the gingival recession defects.
And an additional link to a planned study using Intellicell’s technology for Diabetic Foot Ulcers.
Finally, following is a collection links to official progress reports by the company in regards to successful use of technology for some of the most prominent conditions in today’s healthcare.
IntelliCell Biosciences' Cellular Therapy Treatment Yields Positive Results in Multiple Sclerosis Patients
IntelliCell BioScience Inc. Procedure Enables Norwegian Star Basketball Player to Fully Recover from Patella Tendinitis
IntelliCell Biosciences Stem Cells Used to Successfully Treat Bell's Palsy and Type I Diabetes Patient
Moreover, Dr. Babak Azizzadeh, one of the more prominent doctors in the nation, certainly a superstar in his field, acknowledges the Intellicell’s success.
Babak Azizzadeh, MD, FACS, Director of the Facial Paralysis Institute, comments on the recent treatment of a Bell’s palsy patient with stromal vascular fraction cells.
5) The Patents
IntelliCell BioSciences Receives US Patent for its Stem Cell Extraction Technology
You can find the U.S. patent overview here.
But not only is Intellicell’s technology fully patented in the United States, there are also multiple international patent applications that are pending. Links to European, Australian, Canadian, Thailand and Korean patents can be found in cazual’s post following this link.
It is clear that these patents when granted will open up a whole world of unique revenue-generating possibilities, especially in the countries and jurisdictions where stem cell laws and regulations are much more progressive than in the United States.
And the most important patent of them all, the European one, is one final step away from being granted! The news could come any day now! See the image below (courtesy of Sir Francelote)
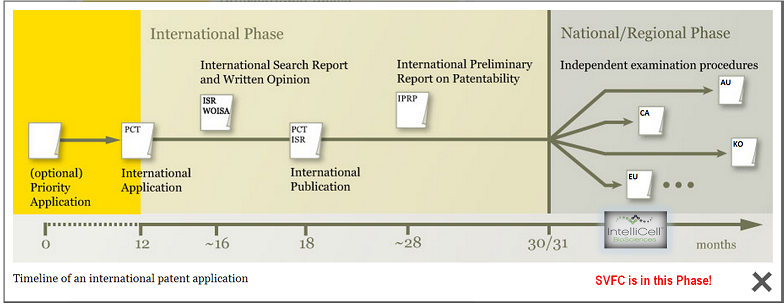
6) The Due Diligence
In December 2013, Dr. Victor returned back from the business trip to a world-renowned hospital in Korea that is actively engaged in stem cell treatments of international celebrities. Related DD and picture confirmation of the trip can be found in this post.
Another impressive picture from the CEO's Korean trip can be seen here. (Source: Dr. Victor's Twitter Account)
More amazing pictures from the Korean trip can be found in this post by click5.
Here is a list of medical staff from CHAUM Global Stem Cell Clinical Trials Center.
Find the pictures of Intellicell's flagship clinic by following this link. Credit: Sir Francelote
Stervc’s comparison and contrast between SVFC and their main competitor CYTX can be seen here.
Some notes on why Intellicell's process without enzymes is superior to that of CYTX, can be read in this post and also here.
You can read my thoughts on why Intellicell's debt is not all that bad by following this link.
The company's business plan is discussed here.
Forbes article discussing Intellicell's advantage.
“A study of IntelliCell’s technology by Millipore, a division of Merck AG of Germany, confirmed that it produced an average of 10 times the number of SVF cells containing adult adipose stem cells from less than which the company believes is used by any of its present competition that use enzymes in their process. The study also showed that the SVF cells produced by IntelliCell contain all of the viable cells that are manufactured by competing technologies that use enzymes, such as Cytori.”
Some calculation and comparison of cell yield from Intellicell and their competitors can be found in this post.
As shown here some of the insiders do believe in this company enough to trust it with couple hundred thousand dollars out of their own pockets.
Here is a link to a post by iHub member LJ Silver about a mystery investor who was willing to provide Intellicell with $500,000 of cold, hard cash.
Sir Francelote provides some quick DD in regards to where Intellicell stands against its competitors in this post.
lee13 shares some good thoughts on the likelihood of a buyout in this post.
Even if the FDA fails to support stem cell industry in the US – multiple international patents (See “The Patents”) will open up countless business possibilities abroad, where regulations in regards to human use of stem cells is much more progressive. And let’s not forget that Intellicell can still explore and capitalize upon less regulated US markets in cosmetics and veterinarian care. In fact, the company has already investigated veterinary market as per this PR.
And let’s not forget that the CEO is NOT getting paid until Intellicell is generating some significant revenues as explained by TheSkunk.
MUST-VISIT LINKS
Most Recent Investor Presentation (February 2013)
Most Recent (7.11.2013) Intellicell's CEO interview (Audio)
Maxim Group Issues Stem Cell Sector Report on IntelliCell BioSciences Based on Treatment Process (July 8, 2013)
The CEO’s Twitter Account
Best of luck!
i.t.m.d.
***Updated: 1/14/2014***
Table of Contents:
1) The Industry
2) The Company
3) The People
4) The Science
5) The Patents
6) The Due Diligence
1) The Industry
Regenerative Medicine (RM) is currently a $15 billion market and is expanding rapidly. In fact, many are calling this LARGELY UNTAPPED market "The Internet of Healthcare," and it is projected to reach $300 billion by year 2020 as patient advocacy groups demanding shift to RM proliferate worldwide (source: US Department of Health and Human Services). 1.2 million patients have been treated to date with RM products and therapies. Currently, 300 private and 50 public companies exist with $4.7 billion in total market capacity. $1.5 billion in worldwide research funding projected to $14 billion in 10 years.
2) The Company
Intellicell is a fully-reporting company currently trading on OTCQB.
The Company's Website
Synopsis:
Intellicell is an emerging leader in regenerative medicine using highly potent stromal vascular fraction cells derived from vasculature of fat tissue via uniquely efficacious technology that is patented in the United States with multiple patents pending internationally. The company is intentioned to enter the large and rapidly growing market of medical conditions where cellular therapies are far more effective than traditional techniques alone.
MOST RECENT SHARE STRUCTURE:
SHARES OUTSTANDING: 316,658,806 as of November 21, 2013 (per latest 10-Q)
AUTHORIZED SHARES: 1,500,000,000
3) The People
The CEO
Dr. Steven Victor, founder and CEO of IntelliCell has been at the forefront of clinical product and process development for over 20 years. The patent pending process that Dr. Victor has developed for IntelliCell™ has been in research for over 4 years. In addition to developing clinical products that are used nationally and internationally in the medical aesthetics field, Dr. Victor is a practicing dermatologist in New York City. He has been a sought after national figure for teaching physicians new clinical techniques worldwide for over 20 years. Dr. Victor has also been featured in national and local media as a clinical subject matter expert in regenerative medicine, medical aesthetics, and dermatology.
Watch the CEO talk about Intellicell below:
Board of Directors
Mr. Michael Hershman
IntelliCell BioSciences Appoints Mr. Michael Hershman as Chairman of the Board of Directors
Mr. Hershman is an internationally recognized expert on matters relating to transparency, accountability, governance, litigation and security. President and CEO of the Fairfax Group, a company he founded in 1983, that has been retained by governments, corporations, law firms and international financial institutions to assist on matters relating to the consult of senior-level officials and/or the entities with which they do business.
More on Mr. Hershman can be found on the website of Fairfax Group by following this link.
Here is a video in which Michael Hershman is awarded Columbia Business School’s 2013 Botwinick Prize in Business Ethics.
I suppose having a renowned business ethics expert and a successful businessman with multiple governmental connections as your BOD Chairman is not a bad thing ;)
Mr. Myron Holubiak
Myron Holubiak is currently President of 1-800-Doctors, Inc. Mr. Holubiak is also Chairman of the Board of Directors of BioScrip, Inc., an infusion and home health company, and Lead Independent Director of Ventrus BioSciences, Inc. From 1998 through 2001, Mr. Holubiak was President of Roche Laboratories, Inc. and previously in his career, he co-founded and served as CEO to Emron, Inc, a strategic marketing firm serving the pharmaceutical industry, which was acquired by Dunn & Bradstreet and IMS Health in 1995. Mr. Holubiak also currently serves on the Board of Trustees for the Academy of Managed Care Foundation.
Mr. Leonard L. Mazur
Mr. Leonard L. Mazur Co-founded Triax Pharmaceuticals, LLC and serves as its Chief Operating Officer. Mr. Mazur also Co-Founded Akrimax Pharmaceuticals in 2007. Mr. Mazur began his pharmaceutical career with Cooper Laboratories in 1971, where he served as a Product Manager for ophthalmology, dermatology and other products. He was responsible for creating and growing the business that was sold to Pierre Fabre, a French dermatology company, in 2002. In 1995, he founded Genesis Pharmaceutical, Inc., and served as its Chairman, Chief Executive Officer from 1995 to 2005 and President.
Mr. Sam Khashman
IntelliCell Biosciences Announces the Addition of Mr. Sam Khashman to Board of Directors
During the past 20 years, Mr. Khashman has successfully directed the creation and launch of 14 products and 3 companies in the healthcare, financial, and manufacturing sector. He currently serves as the President and Chief Executive Officer of Technology Partners, Inc. DBA IMAGINE Software. Mr. Khashman founded the company and created the IMAGINE practice management system that is currently used in 48 States by more than 10,000 physicians. In addition to his involvement in numerous civic and charitable organizations, Mr. Khashman serves on the board of the National Chamber Foundation, the public policy think tank of the U.S. Chamber of Commerce in Washington D.C.
Medical Advisory Board
Dr. James Andrews
IntelliCell BioSciences Inc. Announces James R. Andrews, M.D. Has Joined Its Medical Advisory Board
Doctor James Andrews is one of the founding members of Andrews Sports Medicine and Orthopaedic Center in Birmingham, Alabama. He is also a founder of the American Sports Medicine Institute (ASMI) a non-profit institute dedicated to injury prevention, education and research in orthopaedics and sports medicine. This foundation is recognized as one of the world’s leaders in this field. Doctor Andrews is internationally known and recognized for his skills as an orthopaedic surgeon as well as his scientific and clinic research contributions in knee, shoulder and elbow injury prevention and treatment. In addition, he has made major presentations on every continent, and has authored numerous scientific articles and books.
A list of athletic celebrities treated by Dr. Andrews as of 5 years ago can be found following this link.
Could some of his NEW celebrity patients have been treated with the help of Intellicell’s technology, but not yet publicized?
Dr. Joshua Hackel.
Another member of Andrews Institute on Intellicell’s board of medical advisors is Dr. Joshua Hackel. His profile information can be found here.
Dr. Sydney Coleman
You can read more about Dr. Coleman's extensive stem cell projects funded by the Department of Defense through the Office of the Assistant Secretary of Defense for Health Affairs in this post by lee13.
Note that these fantastic people are just part of the group currently working to move Intellicell’s technology forward. Complete corporate profile for the company can be found following this link. Just Google some of the names not mentioned in this post to see that Intellicell is associated with crème de la crème of medical and scientific communities.
4) The Science
According to this PR, an antibody flow cytometry study of IntelliCell's technology was performed by Millipore, a division of Merck (NYSE: MRK) and found that:
"IntelliCell's proprietary technology yield an average of 10 times (10X) the number of SVF cells containing adult adipose stem cells from less fat (only 2 oz) than that which the Company believes is used by any of its present competition utilizing enzymes in their process. The study also showed that IntelliCells™ contain all of the viable cells that are manufactured by competing technologies that use enzymatic digestion."
The following image presents cell culturing report using Intellicell’s technology:

Moreover, it is because no enzymes are necessary in Intellicell’s process that they are the only public commercial stem cell company in the United States to meet FDA Rule 361 exemption. Under the FDA’s Rule 361, IntelliCells is currently authorized to commercially sell its SVF stem cells in the U.S. The PR announcing the most recent FDA inspection of the company’s flagship lab can be found here. The copy of the FDA’s report can be obtained at this link. Note that most of the observations cited in the report are minor and can be corrected easily. When the FDA spends four days in ANY lab, they WILL find something – it’s just the way the game goes.
Additionaly, the company previously announced that “it has received the results of its independent laboratory audit by Biologic Consultant Group and the audit showed that the new lab at 460 Park Avenue, New York, NY was cGTP compliant.”
Futhermore, as announced here, Intellicell “has been notified according to the FDA validation registration number 3009842420, that its new facility located at 460 Park Avenue, New York, NY 10022 is now registered to recover, process, package, store, and label human cells and tissue products (HCT/P's) such as the IntelliCell autologous stromal vascular fraction cellular product.”
Intellicell Biosciences wins key listing from the FDA
Finally, according to this news release, Intellicell “has been granted a Research Tissue Banking License by the New York State Department of Health.” Which will now allow the company to engage in “the development of autologous and allogenic stromal vascular fraction tissue for researchers around the world.”
A great summary of Intellicell’s technology as compared to current competitors can be found on page 19 of the company’s most recent investor presentation. The visual comparison is presented below:

A list of Intellicell’s current human clinical trials, as officially reported by the company in the latest investor presentation can be found in this post by lee13.
Here is an additional link to a prospective pilot study using Intellicell’s technology on the successful clinical application of SVFC on the gingival recession defects.
And an additional link to a planned study using Intellicell’s technology for Diabetic Foot Ulcers.
Finally, following is a collection links to official progress reports by the company in regards to successful use of technology for some of the most prominent conditions in today’s healthcare.
IntelliCell Biosciences' Cellular Therapy Treatment Yields Positive Results in Multiple Sclerosis Patients
IntelliCell BioScience Inc. Procedure Enables Norwegian Star Basketball Player to Fully Recover from Patella Tendinitis
IntelliCell Biosciences Stem Cells Used to Successfully Treat Bell's Palsy and Type I Diabetes Patient
Moreover, Dr. Babak Azizzadeh, one of the more prominent doctors in the nation, certainly a superstar in his field, acknowledges the Intellicell’s success.
Babak Azizzadeh, MD, FACS, Director of the Facial Paralysis Institute, comments on the recent treatment of a Bell’s palsy patient with stromal vascular fraction cells.
5) The Patents
IntelliCell BioSciences Receives US Patent for its Stem Cell Extraction Technology
You can find the U.S. patent overview here.
But not only is Intellicell’s technology fully patented in the United States, there are also multiple international patent applications that are pending. Links to European, Australian, Canadian, Thailand and Korean patents can be found in cazual’s post following this link.
It is clear that these patents when granted will open up a whole world of unique revenue-generating possibilities, especially in the countries and jurisdictions where stem cell laws and regulations are much more progressive than in the United States.
And the most important patent of them all, the European one, is one final step away from being granted! The news could come any day now! See the image below (courtesy of Sir Francelote)

6) The Due Diligence
In December 2013, Dr. Victor returned back from the business trip to a world-renowned hospital in Korea that is actively engaged in stem cell treatments of international celebrities. Related DD and picture confirmation of the trip can be found in this post.
Another impressive picture from the CEO's Korean trip can be seen here. (Source: Dr. Victor's Twitter Account)
More amazing pictures from the Korean trip can be found in this post by click5.
Here is a list of medical staff from CHAUM Global Stem Cell Clinical Trials Center.
Find the pictures of Intellicell's flagship clinic by following this link. Credit: Sir Francelote
Stervc’s comparison and contrast between SVFC and their main competitor CYTX can be seen here.
Some notes on why Intellicell's process without enzymes is superior to that of CYTX, can be read in this post and also here.
You can read my thoughts on why Intellicell's debt is not all that bad by following this link.
The company's business plan is discussed here.
Forbes article discussing Intellicell's advantage.
“A study of IntelliCell’s technology by Millipore, a division of Merck AG of Germany, confirmed that it produced an average of 10 times the number of SVF cells containing adult adipose stem cells from less than which the company believes is used by any of its present competition that use enzymes in their process. The study also showed that the SVF cells produced by IntelliCell contain all of the viable cells that are manufactured by competing technologies that use enzymes, such as Cytori.”
Some calculation and comparison of cell yield from Intellicell and their competitors can be found in this post.
As shown here some of the insiders do believe in this company enough to trust it with couple hundred thousand dollars out of their own pockets.
Here is a link to a post by iHub member LJ Silver about a mystery investor who was willing to provide Intellicell with $500,000 of cold, hard cash.
Sir Francelote provides some quick DD in regards to where Intellicell stands against its competitors in this post.
lee13 shares some good thoughts on the likelihood of a buyout in this post.
Even if the FDA fails to support stem cell industry in the US – multiple international patents (See “The Patents”) will open up countless business possibilities abroad, where regulations in regards to human use of stem cells is much more progressive. And let’s not forget that Intellicell can still explore and capitalize upon less regulated US markets in cosmetics and veterinarian care. In fact, the company has already investigated veterinary market as per this PR.
And let’s not forget that the CEO is NOT getting paid until Intellicell is generating some significant revenues as explained by TheSkunk.
MUST-VISIT LINKS
Most Recent Investor Presentation (February 2013)
Most Recent (7.11.2013) Intellicell's CEO interview (Audio)
Maxim Group Issues Stem Cell Sector Report on IntelliCell BioSciences Based on Treatment Process (July 8, 2013)
The CEO’s Twitter Account
Best of luck!
i.t.m.d.
This post is my personal opinion. I do not provide investment advice.
i.t.m.d.
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.









