Thursday, March 11, 2010 10:29:51 AM
$heff DD Post on SOMX ($3.90). My personal take into the FDA Decision on 3/21/10. Below are diligent notes that I took from the recent BIO CEO & Investor Conference on FEB 8th. I have listened to it 3 times and have come away confident that the FDA will grant approval and will hold my shares through approval. If the FDA doesn't grant approval then I will be very surprised but it won't be from my lack of DD. It would be a case where the company did everything the FDA guided them to do and still did not approve their drug. There is not much more SOMX can do at this point. I have rarely seen the FDA guide a company as much as it has with Somaxon. The FDA has guided SOMX in the submission process, in their complete response letter, comments regarding the safety of their drug, as well as indications to commit to and not stray from (like sleep maintenance claim & not sleep onset at this point in time). Anyone who hears the Feb 8th BIO CEO conference regarding Somaxon will understand what I am referring to. If you own shares then you should listen to the conference to make your decision. http://www.veracast.com/bio/ceoinvestor2010/enter.cfm
I also remember VNDA Pharmaceutical and how that went higher 80% in one day on a drug, VANAPT, approved for schizophrenia. That was in May 2009. Many thought that would not get approved and shorted the stock down to .80 before it got to over $10.00 on approval. I think that many also believe that Silenor will not get approved even though the stock has climbed in the past month from $2.00 to as high as $485. We will see! I have arranged my notes in a story that speaks to the opportunity that Somaxon has in front of it as a potential new entry into the insomnia market. Those are my personal thoughts are above. Everyone should do their own DD!
DISCLAIMER
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=40119940
_____________________________________________________________
"SOMAXON is staying the FDA course for approval as a new entry in the Insomnia market."
Somaxon is a specialty pharma company focused in the CNS area with their core asset being Silenor for Insomnia.
On January 24th the company met with the FDA to discuss issues with regards to the response letter that they received on December 2009.
http://industry.bnet.com/pharma/10006233/somaxon-pharmaceuticals-nearing-end-of-fda-fatigue-for-silenor/
Following the meeting the agency advised them that they should resubmit the briefing package they provided for that meeting and that by doing so would be considered an adequate response to the complete response letter. Somaxon is now under a Class 1 resubmission with an FDA action Date of March 21st, 2010.
“Class 1 resubmission means the resubmission of an application or efficacy supplement, following receipt of a complete response letter, that contains one or more of the following: Final printed labeling, draft labeling, certain safety updates, stability updates to support provisional or final dating periods, commitments to perform postmarketing studies (including proposals for such studies), assay validation data, final release testing on the last lots used to support approval, minor reanalyses of previously submitted data, and other comparatively minor information.”
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.3
The company has a strategy to achieve their goals. They want to develop & commercialize highly differentiated products, develop a commercial organization, and establish commercial collaborations that drive growth & profitability. If Silenor is approved, the near-term goals are to launch in the 2nd half of 2010 with a partner.
What makes Silenor unique is its different mechanism of action in the insomnia market. It is not a gaba-ergic drug or a melatonin agonist. This could partly explain why it has been described as working so well in the clinic and having a high safety & tolerability profile.
http://www.somaxon.com/pages/silenor.htm
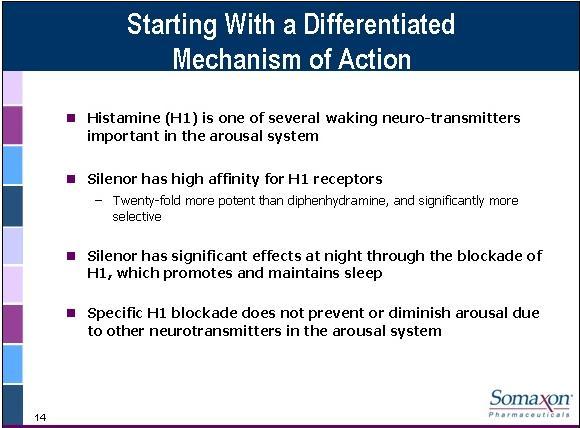
Histamine (H1) is one of several waking neurotransmitters important in the arousal system. Silenor has a high affinity for H1 receptors. H1 is 20 times more potent than diphenhydramine (Benadryl) and significantly more selective. Silenor can promote the qualities of H1 antagonism without the dry eyes, dry mouth, and other toxicities that you may see with a less selective HI. Silenor has significant effects at night through the blockade of H1, which promotes and maintains sleep. Also specific H1 blockade does not prevent or diminish arousal due to other transmitters in the arousal system. The reason that is important is because patients want a drug to promote sleep but not into the next day.
What is important with Silenor is that Somaxon has run 6 well controlled trials in the adult & elderly populations as well as with chronic & transient insomnia. The primary endpoint achieved statistical significance in each of those Phase 2 & Phase 3 studies. Somaxon also ran a comprehensive Phase 1 non-clinical program that was completed. They ran a thorough QT study (results submitted in their NDA last year) that showed no cardiac or QT prolongation at the recommended dose or at the exaggerated does of 50mg. They also ran non-clinical toxicology, drug-drug interaction, and residual effects studies which are required by the FDA all of which were submitted in their NDA.
The CEO stated that the quote from the FDA on Silenor in their complete response letter to Somaxon was:
“There are no adverse events that would preclude approval.”
CEO goes on to state..."As far as safety & tolerability the company is in great shape according to that quote they received in their letter."
The company describes the insomnia market as a “Restless Market. Their are 70 million people in the U.S. that suffer from insomnia and that are self reported insomniacs. The market is underdiagnosed, undertreated, & dissatisfied. Of that 70 million insomniacs, 20% are on a prescription (RX) therapy and of the 14 million pts..2/3 are saying to the market that they are not satisfied with their current treatment options. Over 50 million people in the U.S. use OTC remedies. Sleep maintenance is the primary issue for people who suffer from insomnia. The options that are currently available for patients to have to compromise. Gaba-ergic drugs provide varying degrees of efficacy but involve compromise on tolerability and add the risk of addiction. OTC’s address addiction concern but involve compromise on efficacy & tolerability. Melatonin-receptor agonists address addiction & tolerability concerns but involve a compromise on efficacy.
The efficacy & safety benefits of Silenor would be plenty. Let’s start with the efficacy.
-Silenor met all of their primary endpoints and achieved statistical significance in all 4 Phase III trials. All parameters have been met in elderly & adult populations.
-7-8 hrs of sleep with Silenor. They demonstrated sleep maintenance into the 8th hour of the night which is an important parameter when one thinks about what is clinically meaningful to an insomniac. This is improvement in sleep maintenance, efficiency, and prevention of early morning awakenings
-The drug will not be scheduled & it has no adverse events that exceed placebo to a high degree.
-Silenor’s unique mechanism of action does not have the gaba-ergic related side effects patients might typically see.
-Sleep onset less than 30 minutes is clinically meaningful. The company is not pursuing a sleep onset claim and they were told not to pursue this sleep onset claim by the FDA. They may choose that in the future but for now they are pursuing only a sleep maintenance claim.
With over 70 million prescriptions in the U.S. in 2009, the prescription growth would accelerate with the introduction of a novel, differentiated product. The benefit of Silenor would be that its non-scheduled status would result in significant sampling advantages to providers for Silenor. There is a solid window of opportunity for new treatments as there has been a void of new & differentiated treatments since 2006. There are also no novel compounds expected to be launched in the near-term because there have been clinical and regulatory setbacks for several drugs in development. Somaxon estimates several years before new competition comes to the market so this would be a good time for a new & novel therapy like Silenor to be introduced to the insomnia market. Many patients are anticipating & hoping for a new therapy in the market on March 21st.


I also remember VNDA Pharmaceutical and how that went higher 80% in one day on a drug, VANAPT, approved for schizophrenia. That was in May 2009. Many thought that would not get approved and shorted the stock down to .80 before it got to over $10.00 on approval. I think that many also believe that Silenor will not get approved even though the stock has climbed in the past month from $2.00 to as high as $485. We will see! I have arranged my notes in a story that speaks to the opportunity that Somaxon has in front of it as a potential new entry into the insomnia market. Those are my personal thoughts are above. Everyone should do their own DD!
DISCLAIMER
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=40119940
_____________________________________________________________
"SOMAXON is staying the FDA course for approval as a new entry in the Insomnia market."
Somaxon is a specialty pharma company focused in the CNS area with their core asset being Silenor for Insomnia.
On January 24th the company met with the FDA to discuss issues with regards to the response letter that they received on December 2009.
http://industry.bnet.com/pharma/10006233/somaxon-pharmaceuticals-nearing-end-of-fda-fatigue-for-silenor/
Following the meeting the agency advised them that they should resubmit the briefing package they provided for that meeting and that by doing so would be considered an adequate response to the complete response letter. Somaxon is now under a Class 1 resubmission with an FDA action Date of March 21st, 2010.
“Class 1 resubmission means the resubmission of an application or efficacy supplement, following receipt of a complete response letter, that contains one or more of the following: Final printed labeling, draft labeling, certain safety updates, stability updates to support provisional or final dating periods, commitments to perform postmarketing studies (including proposals for such studies), assay validation data, final release testing on the last lots used to support approval, minor reanalyses of previously submitted data, and other comparatively minor information.”
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.3
The company has a strategy to achieve their goals. They want to develop & commercialize highly differentiated products, develop a commercial organization, and establish commercial collaborations that drive growth & profitability. If Silenor is approved, the near-term goals are to launch in the 2nd half of 2010 with a partner.
What makes Silenor unique is its different mechanism of action in the insomnia market. It is not a gaba-ergic drug or a melatonin agonist. This could partly explain why it has been described as working so well in the clinic and having a high safety & tolerability profile.
http://www.somaxon.com/pages/silenor.htm
Histamine (H1) is one of several waking neurotransmitters important in the arousal system. Silenor has a high affinity for H1 receptors. H1 is 20 times more potent than diphenhydramine (Benadryl) and significantly more selective. Silenor can promote the qualities of H1 antagonism without the dry eyes, dry mouth, and other toxicities that you may see with a less selective HI. Silenor has significant effects at night through the blockade of H1, which promotes and maintains sleep. Also specific H1 blockade does not prevent or diminish arousal due to other transmitters in the arousal system. The reason that is important is because patients want a drug to promote sleep but not into the next day.
What is important with Silenor is that Somaxon has run 6 well controlled trials in the adult & elderly populations as well as with chronic & transient insomnia. The primary endpoint achieved statistical significance in each of those Phase 2 & Phase 3 studies. Somaxon also ran a comprehensive Phase 1 non-clinical program that was completed. They ran a thorough QT study (results submitted in their NDA last year) that showed no cardiac or QT prolongation at the recommended dose or at the exaggerated does of 50mg. They also ran non-clinical toxicology, drug-drug interaction, and residual effects studies which are required by the FDA all of which were submitted in their NDA.
The CEO stated that the quote from the FDA on Silenor in their complete response letter to Somaxon was:
“There are no adverse events that would preclude approval.”
CEO goes on to state..."As far as safety & tolerability the company is in great shape according to that quote they received in their letter."
The company describes the insomnia market as a “Restless Market. Their are 70 million people in the U.S. that suffer from insomnia and that are self reported insomniacs. The market is underdiagnosed, undertreated, & dissatisfied. Of that 70 million insomniacs, 20% are on a prescription (RX) therapy and of the 14 million pts..2/3 are saying to the market that they are not satisfied with their current treatment options. Over 50 million people in the U.S. use OTC remedies. Sleep maintenance is the primary issue for people who suffer from insomnia. The options that are currently available for patients to have to compromise. Gaba-ergic drugs provide varying degrees of efficacy but involve compromise on tolerability and add the risk of addiction. OTC’s address addiction concern but involve compromise on efficacy & tolerability. Melatonin-receptor agonists address addiction & tolerability concerns but involve a compromise on efficacy.
The efficacy & safety benefits of Silenor would be plenty. Let’s start with the efficacy.
-Silenor met all of their primary endpoints and achieved statistical significance in all 4 Phase III trials. All parameters have been met in elderly & adult populations.
-7-8 hrs of sleep with Silenor. They demonstrated sleep maintenance into the 8th hour of the night which is an important parameter when one thinks about what is clinically meaningful to an insomniac. This is improvement in sleep maintenance, efficiency, and prevention of early morning awakenings
-The drug will not be scheduled & it has no adverse events that exceed placebo to a high degree.
-Silenor’s unique mechanism of action does not have the gaba-ergic related side effects patients might typically see.
-Sleep onset less than 30 minutes is clinically meaningful. The company is not pursuing a sleep onset claim and they were told not to pursue this sleep onset claim by the FDA. They may choose that in the future but for now they are pursuing only a sleep maintenance claim.
With over 70 million prescriptions in the U.S. in 2009, the prescription growth would accelerate with the introduction of a novel, differentiated product. The benefit of Silenor would be that its non-scheduled status would result in significant sampling advantages to providers for Silenor. There is a solid window of opportunity for new treatments as there has been a void of new & differentiated treatments since 2006. There are also no novel compounds expected to be launched in the near-term because there have been clinical and regulatory setbacks for several drugs in development. Somaxon estimates several years before new competition comes to the market so this would be a good time for a new & novel therapy like Silenor to be introduced to the insomnia market. Many patients are anticipating & hoping for a new therapy in the market on March 21st.
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.







