Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Just echoing your great finds which made my week!!
Sales Track shows steep sales declines for BioElectronics.😎
BIEL #1 on the No Money Board.😎
Misinformation and Disinformation. Anti-Biel BS Artist #2.
That's right, GO BIEL!!
Great information was shared today by Hawk and Srin about developments that can potentially change the future of Biel in a short amount of time.
Would it ever occur to you to acknowledge a message of positive news with a simple "Thank you" or any other form of appreciation? Probably not, but I'm optimistic.
P.s. Studies show that people who are focused on the present are happier than those who focus on the past. Try it.
Hawk, good job on DD, hope there is a lil substance.
Actipatch on special Oct-Dec 2024
https://arrienel.co.za/family-basket-deals/
What information was false? Did they not FAIL at all that was stated? Were they ever profitable in any year in business? Where is Mundipharma? Are they selling in UK? Where is Allay selling in USA? (They are NOT selling because they FAILED getting USA FDA clearance). And drum roll please - - - - - where is the biggest of all JOKES this company announced to all you stockholders? Yes, where is SYNERGY???!!!
But anyway --- GO KELLY - GO BIEL!!! LOL!
Srin and Hawk: Wow, great work. Thanks again for sharing!
I'm very impressed by the information you shared today. Obviously a lot of work has been done, over the course of the year, by the people involved. Taiwan gets it!! Maybe one day, the US may too.
Thanks for sharing, Hawk!!
BIEL'S Nite Capitals pump squads in full swing.😎
Seriously folks, no kidding, and BTW BIEL is dead.
I'm very impressed by the information you shared today. Obviously a lot of work has been done, over the course of the year, by the people involved. Taiwan gets it!! Maybe one day, the US may too.
Thanks for sharing, Hawk!!
Very nice Srin!
I've read it several times since this morning.
And if you know me...., I've looked up every name, company or anything in that article.
The Next Big Thing, thanks Hawk
According to survey statistics, nearly 50% of Chinese people, or about 10 million people, suffer from pain! To this end, Eugenics Pharmaceutical Factory has strategically cooperated with Avro Biomedical Consultants to launch today (7th) the "Antong Pain Relief Ring"(ActiPatch), a portable secondary medical device exclusively imported from the United States. It was confirmed by research from the Department of Anesthesia and Pain at National Taiwan University. The pain management and quality of life of patients have been significantly improved, and many large medical institutions across Taiwan have successively applied for the use of Pain Relief Ring.
According to statistics from the National Health Insurance Administration, the number of painkillers used by people in Taiwan exceeds 900 million tablets a year, ranking first among health insurance drugs. In addition, people use 438 million sore patches a year, which shows that people are very interested in treating pain. eager.
According to the definition of the International Pain Society, pain that lasts for more than 3 months is considered chronic pain. According to surveys, nearly 50% of Chinese people, or about 10 million people, suffer from pain every week. The top three are muscle soreness, headache, and joint pain.
According to statistics from the National Health Administration, about 4 million people in Taiwan suffer from chronic pain problems, which are more likely to occur in the elderly and women. These patients are often in pain that makes them helpless, suffer from pain that makes them unable to sleep, and even seriously affects their quality of life.
Sun Wei-ren, a specially appointed part-time attending physician at National Taiwan University and current director of the Pain Center of Cathay General Hospital, said that pain is the body's self-protection mechanism. When pain attacks frequently or persists, you should seek assistance from a pain specialist to find out the real cause and actively correct the pain. Treatment and pain management can help improve the quality of life.
Sun Weiren emphasized that for chronic pain that has caused sensitization of the central nervous system, in addition to increasing exercise, improving lifestyle, and medications or interventional treatments prescribed by medical personnel, other better auxiliary treatments still seem to be needed.
Qian Leli, director of the Orthopedics Department of Clarification Hospital, said that painkillers are usually used to relieve sudden and short-term pain. If you want to completely solve the problem, you still need to find out the root cause of chronic pain, and according to the doctor’s diagnosis, you can cooperate with non-drug treatments, such as cold therapy. , hot compress, stretching, exercise rehabilitation therapy or other non-invasive medical equipment to help relieve pain, reduce medication, and also reduce the burden of analgesic drugs on the liver and kidneys.
Send feedback
Dr. Chen Junren, deputy general manager of Eugenics Pharmaceutical Factory, said that Eugenics Pharmaceuticals, which was famous for producing "heart-strengthening" heart drugs in 1945, strategically cooperated with Avro Biomedical Consulting Company to exclusively introduce a drug called "Antong and Relief Ring" from the United States. "A portable secondary medical device that uses pulsed electromagnetic fields (PEMF) approved by the United States to act on deep tissues of the body to help reduce pain from musculoskeletal or nervous system-related diseases, and can effectively relieve chronic pain caused by central nervous system sensitization. .
Chen Junren said that the results of the "Long-acting Electromagnetic Pulse Chronic Pain Relief Study" conducted by Professor Sun Weiren of the Department of Anesthesiology and Pain at National Taiwan University, physician Liu Zhimin and their research team showed that for patients with chronic pain due to herpes zoster pain and low back pain in outpatient clinics, comfort can Pain rings have achieved significant benefits in terms of pain management and quality of life for patients, and "pain relief rings" have become another choice for pain experts.
As a medical treatment method, "Electromagnetic Pulse Pain Relief Treatment" has been approved by the Health Bureau of the Taipei City Government and is officially used at National Taiwan University Hospital. It is used with the professional judgment of doctors and according to the cause and severity of pain, "An Pain Relief" is worn according to the doctor's instructions. "Pain Relief Ring" is used to help relieve pain associated with musculoskeletal or neurological conditions.
Following National Taiwan University, Taipei Tzu Chi Medical Center and Mackay Medical Center in the north, Ching Ching Hospital and Lin Hsin Hospital in the central part, and the National Army Beitou and Hsinchu Hospital have successively applied for the use of pain relief rings to benefit more patients suffering from chronic diseases. Patients suffering from pain.
Chen Junren, who is also a pharmacist himself, said that pain management is a very important part of medical care. As Taiwan enters a super-aged society and the number of patients with various types of pain increases year by year, the selection and long-term use of drugs will cause changes in liver and kidney function. Under the influence, the Pain Relief Ring is drug-free and can be used as an auxiliary treatment method to relieve pain 24 hours a day. It is a new choice to help patients stay away from pain!
Purchasing items in the online world has no borders, but health authorities remind that if you purchase products under medical device management online in foreign shopping malls, because the products have not obtained the medical device license approved by China, there may be risks in terms of safety, effectiveness and quality. There are doubts. If people buy on their own and encounter problems with use, they may have no way to seek compensation. Even if products without an approved "import license" are intercepted by customs, the purchaser will be fined by the Health Bureau, the goods may be confiscated, and the goods may be confiscated. Required to pay scrap disposal fees.
Health authorities remind the public to choose medical equipment with brands approved by the Ministry of Health and Welfare and recommended for use by medical institutions that specialize in pain management. They should not purchase products from unknown sources from overseas online shopping malls to avoid violating the law.
More reports from China Times News Network
Is the loss of TSMC’s mature manufacturing processes actually enriching SMIC? Structural worries will erupt within 10 years Lu Xingzhi: Can we and UMC jointly defend against foreign enemies?
The eldest wife launches a counterattack! She took 300 million yuan in stock from her husband to do something else, and the mistress’s illegitimate daughter was slapped with triple inheritance tax
Finally got through the hard times! Fed cuts interest rates, U.S. investment banks are bullish
https://tw.stock.yahoo.com/news/%E5%9C%8B%E4%BA%BA%E9%A3%BD%E5%8F%97%E7%96%BC%E7%97%9B%E4%B9%8B%E8%8B%A6-%E5%84%AA%E7%94%9F%E8%A3%BD%E8%97%A5%E8%87%AA%E7%BE%8E%E5%9C%8B%E5%BC%95%E9%80%B2-%E5%AE%89%E7%96%BC%E8%88%92%E7%97%9B%E7%92%B0-%E6%88%90%E6%85%A2%E6%80%A7%E7%96%BC%E7%97%9B%E6%96%B0%E8%A7%A3%E6%96%B9-122146817.html
Honorable Mention, Post-Of-The-Day #2, Must Read.
Honorable Mention, Post-Of-The-Day #1, Must Read.
Official!! Post Of The Day Award!! Must Read!!
Use Google Translator.
BIEL #5 on the Breakout Boards and #12 Most Read the past hour.
Massive Display Of Misinformation and Severe Slant There.
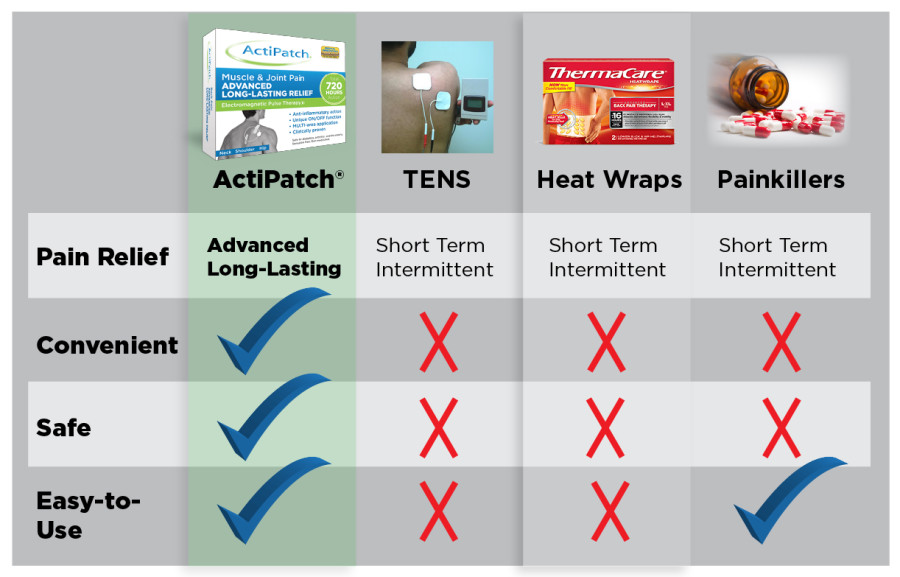
Good reminder Hawk, Ibuprofen use can result in Kidney damage and Acetaminophen use can damage your Liver, ActiPatch is Side Effect Free
Ibuprofen can cause AKI, which can range from minor kidney function loss to complete kidney failure. AKI can occur when ibuprofen reduces blood flow to the kidneys, which can deprive them of oxygen. Symptoms of AKI include producing less urine, swelling in the arms, legs, or feet, nausea, confusion, and weakness.
Taking too much acetaminophen can lead to overdose and severe liver damage. Symptoms of acetaminophen overdose may include nausea, vomiting, abdominal pain, confusion and jaundice (yellow skin and eyes).Feb 1, 2024
Alright Hawk, Srin said "Ask Hawk".
I was wondering if the Hospitals mentioned were in some way doing direct business with Biel or having some dealings with them??
I would think this would be a major play for Biel.
Okay, I understand.
Better check in with Hawk, I would think the new ActiPatch distributor, Daniel Yang, VP of Aphrodite Bio Medical and Arthur Chen, VP of Yusheng Pharmaceutical, are involved unless BIEL has a separate RRx distributor in Taiwan for Hospitals. The BIEL website is down so I can't check the distributor list.
https://www.facebook.com/story.php?story_fbid=1089700813161289&id=100063641406505&rdid=JS5jOuAJD3M4m1po
For the sake of understanding clearly, the 2 Hospitals that you are referring to are having direct business dealings with Biel??
Yeah, THAT'S a huge market that should push this over the TOP! They cannot sell enough product for the number one HUMAN PAIN - BACK PAIN but PLP will do the trick! They have the golden clearance from the USA FDA for ALL MUSCLE & JOINT PAIN for YEARS and FAILED to make this company profitable. They FAILED with what would have been a big market when ALLAY got denied. They FAILED selling, when they got clearance in the UK. They FAILED with MUNDIPHARMA. And they failed trying to convince anyone that this announcement they made in 2022, was true: https://investorshub.advfn.com/uimage/uploads/2023/8/20/yeqevsynergy.jpeg
But please, keep those 'pearls of wisdom' coming! LOL!
Have a great night!
WOW!! Starting To Percolate!!
The MacKay Medical Center is also impressive in size
3,500 Hospital Beds
8,800 Employees
372,000 Patients each month
Successively applied for the use of ActiPatch Pain Relief Ring.
I was thinking more about the 'Phantom Limb Pain is not Real Pain' BS and the 'BIEL Money Back Guarantee is a Scam' BS
Imagine if they tap 10% or even greater- -
>>>The 'Usual Suspects' would like nothing better than to have their version of the 'BIEL World' go unchallenged, Not Going to Happen!<<<
There is NO 'challenging' those financial statements. They ARE UGLY!! Honestly, I don't know which statement is worse, the income statement that shows another NET LOSS and declining sales or the balance sheet that shows an asset to liability ratio of 1/115. Then there is the 'cash on hand' of $4686 and 'current' liabilities of $4,093,176 ('current' means due in one year). That's a little concerning, no? PLUS it shows $12,529,631 in notes that have all come due but remain unpaid with a TOTAL of liabilities due of $16,671,419.
That's pretty sad, isn't it and hard to 'challenge'. Thus trading one and two ticks from ROCK BOTTOM!
Have a great night!
Great news Hawk, the National Taiwan Hospital is enormous
5,000 Hospital Beds
12,000 Employees, 1,300 of those are Physicians
8,000 Outpatient Visits Daily
ActiPatch pain Relief Ring is already in use.
Yeah Right, I will shoot down a rouge post only once and give it a pass the other 99 times it shows up on the Board.
The 'Usual Suspects' would like nothing better than to have their version of the 'BIEL World' go unchallenged, Not Going to Happen!
Taiwan!! This Is Significant. Great Find.
BIEL #17 on the Breakout Boards and #22 Most Read the past hour.
Great work again Hawk! Taiwan is a great market for our products!!!
This trash management ever gonna do anything besides lose shareholder value and seek revenge on holders?
https://tw.stock.yahoo.com/news/%E5%9C%8B%E4%BA%BA%E9%A3%BD%E5%8F%97%E7%96%BC%E7%97%9B%E4%B9%8B%E8%8B%A6-%E5%84%AA%E7%94%9F%E8%A3%BD%
E8%97%A5%E8%87%AA%E7%BE%8E%E5%9C%8B%E5%BC%95%E9%80%B2-%E5%AE%89%E7%96%BC%E8%88%92%E7%97%9B%E7%92%B0-%E6%88%90%E6%85%A2%E6%80%A7%E7%96%BC%E7%97%9B%E6%96%B0%E8%A7%A3%E6%96%B9-122146817.html
After seeing post #335033 from Hawk - both photo's sure look like Bioelectronics Actipatch!
Could Eugenics Pharmaceuticals ?????????????????????????????????
The view looking forward is quite different than the view looking behind. Nearly $39 million tax-free profits will accelerate the pps upward after revenues exceed $1.5 million.
$1.5 - 2 million = break even, cash flow positive: pps = .01.
Each additional $2.5 million/year should result in an additional .01 increase in pps. Each of the following potential catalysts could result in more than $2.5 million annual revenue:
1) Veterans Administration ($5 mil?)
2) Veterinary company contract ($5 mil?)
3) Two new large company contract rumors ($5 mil?)
For revenue = $15 milllion, resulting pps =
.07 (P/E = 100)
.14 (P/E = 200)
.21 (P/E = 300)
RFK post. Also big pharma freakout , could push them into alternative medicine to show Trump and RFK that they are willing to bend so things don't get too drastic...
President Trump has asked me to do three things:
— Robert F. Kennedy Jr (@RobertKennedyJr) November 6, 2024
1. Clean up the corruption in our government health agencies.
2. Return those agencies to their rich tradition of gold-standard, evidence-based science.
3. Make America Healthy Again by ending the chronic disease epidemic. pic.twitter.com/WHMOsD0CiI
Nope…but a nice speech nonetheless
You pointed out that someone has been posting the same thing for years and implied that that fit the definition of insanity.
I pointed out that you have been responding to those post for almost as many years, and implied that the irony was lost on you.
That’s all. It was actually pretty funny if you stand back and look at it in an unattached manner.
Hey Srin!
Check out the posts from this morning with the attachment there's a lot going on in Taiwan
So, your opinion is that new readers of the BIEL Board should have no background on a poster that has been trashing BIEL for 13 years/28,000 posts, often posting blatant fabrications like 'Sales Track', 'Andy told me RS coming in days', 'BIEL headed to BK', got it.
I believe that everyone is entitled to an opinion but when they present that opinion as a fact, not the case of an error but over and over, they need to be called out for it. And no, having a tag line that says, 'Everything I say and write is my opinion and my opinion only', does not exonerate one from presenting BS as a true statement. That is where my moral compass points, but obviously compasses vary.
|
Followers
|
1074
|
Posters
|
|
|
Posts (Today)
|
1
|
Posts (Total)
|
335088
|
|
Created
|
05/18/05
|
Type
|
Free
|
| Moderators misanthrope Capsule JustGoDeep toohot JNdouble1 gimmee greenbacks | |||
PHOENIX, June 13, 2018 (GLOBE NEWSWIRE) -- Uptick Newswire welcomed Andy Whelan, President of BioElectronics Corporation (OTC Pink:BIEL) ("the Company") back onto the "Stock Day" podcast. Andy spoke with Everett about their progress in the US market, UK market, the new product, the US FDA, and new distributors and potential partnership opportunities.
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: ActiPatch(R) Therapy, over-the-counter treatment for back pain and other musculoskeletal complaints; RecoveryRx(R) Devices for chronic and post-operative wound care; Allay(R) Menstrual Pain Therapy. For more information, please visit http://www.bielcorp.com/
The Company is currently pursuing multiple US partnership opportunities that are partially contingent on additional FDA clearance for the treatment of musculoskeletal pain, back pain, knee pain, muscle, hip, carpal tunnel, etc. Whelan said,"Patience is key throughout this process. It's going as well as it can." Additionally, the Company anticipates solidifying deals with two new major distributors in the Asian and Eastern European markets.
The Company's product development and regulatory clearances are going well. Now, with pre-submission clearance, the 510(k) relief of musculoskeletal pain market clearance application will be filed by the end of June.
The US FDA has recently implemented an Innovation Program seeking "Breakthrough Devices" to Prevent and Treat Opioid Use Disorder. Opioids are routinely used for post-operative pain. Our United Kingdom ActiPatch User Registry accounts that 71% of opioid customers report a moderate (50%) to complete (100%) elimination in opioid medication use. The Company plans to submit its post-operative pain relief market clearance application into this program. The FDA's assistance, participation, and approval will provide credence and accelerate product market acceptance.
The Company has begun shipments of its new single box kinesiology tape product to be used for back, knee, hip, and muscles and joints in lieu of the back wrap, knee wrap and muscle and joint adhesives. The kinesiology tape is easier to use, reduces product costs, and retail shelf space requirements.
The Company is transitioning its United Kingdom over-the-counter sales and marketing from the front of the store to behind the counter to expand product availability from a few stores to all 14,000 pharmacies. Most pharmacies obtain daily product deliveries and most people in the UK qualify for free prescriptions.
Whelan is confident in the direction the Company is moving, and cites the unique nature of their products as a factor that helps the Company stand out in the global medical electronics industry. Whelan then said, "This is one busy little Company."
"Stock Day" host Everett Jolly stands by his assessment from his April interview with the company that the stock is very undervalued. BioElectronics Corporation trades on the OTC Pink market under the ticker symbol BIEL. Shares are currently selling at 0.0037, up over 100% since April.
For more details about the Company's recent FDA meetings, potential partnership opportunities, and attempts to combat the ongoing opioid crisis, follow the link below to hear the full interview.
https://upticknewswire.com/featured-interview-ceo-andrew-whelan-of-bioelectronics-corp-otc-pink-biel-3/ ;
About BioElectronics Corporation
BioElectronics develops and manufactures unique nonprescription affordable neuromodulation medical devices. The Company's technology platform is for the treatment of central sensitization, which is now widely accepted as the physiological explanation for many neurological disorders, and specifically chronic pain. Central sensitization is difficult, if not impossible, to address through pharmacotherapy without having a detrimental impact on normal physiologic function. Moreover, pharmacological and masking therapies (creams, heat patches, TENS etc.) have limited effectiveness for chronic pain due to their transient nature. BioElectronics' electromagnetic stimulation therapy has the distinct advantage of being safe, affordable, long lasting, and effective pain relief.
Contact: Paul Knopick, 940.262.3584, pknopick@eandecommunications.com
FREDERICK, MD., May 31, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), maker of advanced medical devices announces that B. Braun Ltd, of Sheffield, UK has recently completed development of its same day surgical TOTAL Pathway program for joint replacements which includes BioElectronics medical devices. The program is being launched by B. Braun’s UK […]
FREDERICK, MD, May 29, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com reports that it has cancelled today’s scheduled US Food and Drug Administration meeting. “The FDA’s response to our questions, suggestions, and instructions are more than adequate for us to proceed with filing of the formal 510(k) Market Clearance Application,” stated Ian […]
FREDERICK, MD , May 11, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to report on the outcome of its Pre-Submission meeting on May 9th, 2018 with the US Food and Drug Administration (US FDA). The FDA’s Pre-Submission Program is designed to organize and give guidance and feedback on clinical data and what […]
FREDERICK, MD, May 02, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to announce that two meetings have been scheduled with the US Food and Drug Administration (FDA) to seek expanded over-the-counter (OTC) clearances for their drug-free, wearable medical devices. The first meeting will take place on May 9th, 2018 to […]
PHOENIX, April 26, 2018 (GLOBE NEWSWIRE) — Uptick Newswire was thrilled to have Keith Nalepka, VP of Marketing & Sales at BioElectronics (OTC Pink:BIEL) (“the Company”), return to the “Stock Day” podcast. The company has been working hard lately to expand the United Kingdom market with B. Braun in the surgical market and the product […]
FREDERICK, MD, March 19, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com is pleased to announce the commencement of a clinical study investigating the efficacy of its RecoveryRx® device for postoperative pain management and recovery following total knee arthroplasty surgery. Currently there are 700,000 total knee replacement surgeries in the US alone and are projected to grow 673% to 3.5 million procedures per […]
PHOENIX, Jan. 22, 2018 (GLOBE NEWSWIRE) — The Uptick Newswire “Stock Day” podcast keeps investors up to date on the latest penny stock news by bringing transparency in the micro-cap side of the market. Connect with “Stock Day” and to over 600+ CEO interviews on the OTC, Pink Sheets and micro-cap news from around the world […]
FREDERICK, MD, Jan. 09, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC Pink: BIEL), the maker of advanced wearable consumer pain management medical device, ActiPatch, announced today that CARE Pharmacies has committed to introducing ActiPatch, Musculoskeletal Pain Therapy medical device to its patients. “With the current opioid crisis here in the United States, as pharmacists, we […]
FREDERICK, Md., Jan. 04, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com makes wearable, drug-free chronic pain therapy medical devices. It is pleased to announce that the UK’s government funded public health service, National Health System (NHS), has approved our application to cover and pay for ActiPatch® Musculoskeletal Pain Therapy. The NHS estimates that almost half […]
FREDERICK, MD, Dec. 12, 2017 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC Pink: BIEL), the maker of advanced wearable consumer pain management medical device, ActiPatch, announced today the launch of ActiPatch for Over-The-Counter, drug-free pain relief as an alternative to Opioids. ActiPatch is approved by the FDA for Over-The-Counter sales for the adjunctive treatment of pain […]
BioElectronics Receives FDA Pre-Submission Approval for its Relief of Musculoskeletal Pain Market Clearance Application
FREDERICK, MD , May 11, 2018 (GLOBE NEWSWIRE) -- BioElectronics Corporation (BIEL) , www.bielcorp.com is pleased to report on the outcome of its Pre-Submission meeting on May 9th, 2018 with the US Food and Drug Administration (US FDA). The FDA’s Pre-Submission Program is designed to organize and give guidance and feedback on clinical data and what regulatory pathway should be followed to get market clearance. The focus of the May 9 pre-submission meeting was in discussing the FDA’s feedback on the clinical outcomes and statistical techniques used in reporting the back-pain study.
Upon reviewing pre-submission information on the ActiPatch® back-pain study (https://clinicaltrials.gov/ct2/show/NCT03240146), the FDA provided positive feedback on the clinical results, and guidance on a 510(k) submission to obtain expanded market clearance for over-the-counter (OTC) treatment of musculoskeletal pain. This would make ActiPatch available as a drug-free, safe, pain relief option for the 126 million Americans (one in two adults) who are suffering with some form of musculoskeletal pain. While ActiPatch is already FDA cleared for treatment of pain from knee osteoarthritis (25 million) and plantar fasciitis (1 million annually), expanded market clearance would allow additional products for the back (42 million), neck (19 million), hip (9 million), shoulder (11 million), carpal tunnel (12 million) and many other musculoskeletal complaints.
The company was represented by their clinical R&D team comprising: Sree Koneru, Ph.D., VP Product Development, Ian Rawe, Director Clinical Research of BioElectronics (BIEL), Kenneth McLeod, Ph.D. Director of Clinical Science and Engineering Research, State University of New York at Binghamton and Richard Staelin, Ph.D. Professor Duke University. Dr. Koneru, who led the discussion, expressed confidence in the FDA’s constructive feedback. “We are pleased that the FDA viewed our data and statistical methods favorably. They have provided guidance on how to combine the back-pain study results, along with our previously cleared 510(k), into a single 510(k) submission for obtaining expedited expanded market clearance.”, he said.
An additional pre-submission meeting is scheduled with the FDA on May 29th, 2018 to seek expanded indications in a separate application for OTC treatment of pain and edema following surgical procedures for its RecoveryRx® medical device.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: ActiPatch® Therapy, over-the-counter treatment for back pain and other musculoskeletal complaints; RecoveryRx® Devices for chronic and post-operative wound care; Allay® Menstrual Pain Therapy. For more information, please visit www.bielcorp.com
BioElectronics VP of Sales Discusses Pre & Post-operative Pain Relief Surgical Program in "Stock Day" Interview
PHOENIX, April 26, 2018 (GLOBE NEWSWIRE) -- Uptick Newswire was thrilled to have Keith Nalepka, VP of Marketing & Sales at BioElectronics (OTC Pink:BIEL) ("the Company"), return to the "Stock Day" podcast. The company has been working hard lately to expand the United Kingdom market with B. Braun in the surgical market and the product opportunity from the recent National Health Service payment approval for its ActiPatch® medical device.
BioElectronics is with B. Braun Medical, Ltd.UK to initiate the "Total Pathways Program" program into B. Braun's 26 geographic markets. The program is for pre-habilitation (treatment that happens prior to surgery) and post-operative recovery. This new program will benefit the majority of the 160,000 UK annual knee, hip, or joint replacements patients. According to Keith, BioElectronics is not just trying establishing a brand, but "to establish the process to a standardize care for pre and post-operative pain."
The company is immensely proud of their team for getting their concept through to the National Health Service (NHS) in the UK. In addition to successes here, the company is hoping to utilize their overseas success by applying data to their US-based efforts.
There are some great potential partnerships currently being discussed at BioElectronics, with the closing of a significant deal just on the horizon.
BioElectronics Corp is approaching their upcoming FDA meetings with confidence. "We have the data to make a very strong case for both broader musculoskeletal and post-operative pain relief indications," said Nalepka. "It's an exciting time for the company, and a really cool little product."
For more details on the Total Pathways Program, news about a partnerships, and information about the company's upcoming meetings with the FDA, follow the link below to the full interview.
https://upticknewswire.com/featured-interview-vp-of-sales-keith-nalepka-of-bioelectronics-corp-otcpink-biel-3/ ;
About BioElectronics
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
Contact: Paul Knopick, 940.262.3584, pknopick@eandecommunications.com
Safe Harbor Statement
Certain statements contained herein are "forward-looking statements," (as defined in the Private Securities Litigation Reform Act of 1995). BioElectronics Corp. cautions that statements and assumptions made in this news release constitute forward-looking statements; the company makes no guarantee of future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. These statements may address issues that involve significant risks, uncertainties, and other estimates made by management. Actual results could differ from current projections or implied results. BioElectronics Corp. undertakes no obligation to revise these statements following the date of this news release.
About Uptick Newswire
Uptick Newswire is a private company reaching out to the masses keeping investors and shareholders up to date on company news and bringing transparency to the undervalued, undersold, micro-cap stocks of the market and is the sole producer of the Uptick Network "Stock Day" Podcast. The Uptick Network "Stock Day" Podcast is an extension of Uptick Newswire and has recently launched the Video Interview Studio located in Phoenix, Arizona.
Contact Information
Uptick Newswire LLC
Everett Jolly, CEO/Founder
602-441-3474
10000 N. 31st Avenue C307 Phoenix, AZ 85051
info@upticknewswire.com
www.upticknewswire.com
| Release #:812-170554-rl-1141231: |
| BioElectronics Up-Date |
| Dear Fellow Shareholders, This letter is to report on our recent progress in marketing and product development. We have established the following:
We are continuing to solicit new international and domestic sales and marketing partners for distribution and licensing, while strengthening our relationship with existing partners. To aid in this effort we have engaged an accomplished sales and marketing pharmaceutical consultant to assist in introduction and negotiations of sales and marketing partnerships. US Market Clearance ActiPatch®, Musculoskeletal Pain Therapy We are in the process of expanding the US market clearance from relief of osteoarthritis of the knee pain and plantar fasciitis. Our goal is to obtain general clearance for musculoskeletal pain. To achieve this, we have submitted a chronic back pain clinical study to the US FDA as a formal Pre-Sub. The initial meeting with the FDA is tentatively scheduled for May 9th. Prior guidance from the FDA indicated that a third clinical study was required before expanding the current market indications to cover all musculoskeletal pain. We are hopeful that the process will be completed rapidly, however we will not have a good estimate of this market clearance date until the completion of the May 9th meeting. Postoperative Recovery and Pain We have submitted a formal Pre-Sub to the US FDA for guidance in obtaining market clearance for the palliative treatment of postoperative pain and edema. The application is supported by postoperative clinical studies on breast augmentation and caesarean section. Marketing Expansion We believe that expanded market clearance from the FDA will enhance the market value and attractiveness of the ActiPatch product line. Our economic and clinical studies for the National Health Services allowed us to gain a drug tariff listing for reimbursement in England and Wales allowing the following:
The ActiPatch registry studies report that people buy the device because it is effective, drug-free, has no harmful side effects and overall improves quality of life. We are continually working to solve existing minor issues with the use of ActiPatch. To improve the product, we are introducing custom-design kinesiology tape strips to attach the device to the body. Aside from improving user comfort, this allows us to promote a single multi-purpose box with the added benefit of reducing retail shelf space. We believe this will then allow us to sell the 7-day trial devices from pharmacy shelves. B. Braun UK This is an important development for BioElectronics. B Braun has been using RecoveryRx to improve recovery following joint replacement for many years, although in limited surgeries. However, new advances in day surgery for joint replacement have expanded our opportunity. B. Braun has recently completed a pilot program in the UK evaluating day for joint replacement. The United Kingdom's Group day surgery program for knee and hip replacement patients and is now being implemented on a national basis. Currently there are 80,000 procedures per year involving B Braun. The "fast-track" same day hip and knee replacements are being supported by hospital physiotherapy teams visiting the patient at home and communicating using a special wireless technology tablet. Upon hospital discharge, each patient will be given an ActiPatch medical device and a prescription for 6 additional devices to help accelerate recovery and mitigate the postoperative pain. A similar program is in development for spinal surgeries. On an international level, B. Braun's 54,000 employees are in 60 countries and are separated into additional divisions for hospital care, surgical products, outpatient care, and home care services. It is anticipated that B. Braun's German Group will roll out the same day hip and knee replacement program in the next few months.
Smart Insole – Heel Pain Relief We have redesigned the Smart™ Insole plantar fasciitis insole to enhance user comfort. We are awaiting a written License and Supply Agreement for the product. Clinical Research to Expand Market Clearance and Acceptance Allay®, Menstrual Pain Therapy 28% of women are in the menstrual phase of life and 60% have moderate to severe menstrual pain, or 17% of women. Previously our medical devices were classified as high risk class III by the US FDA. While our existing pilot clinical study for menstrual pain reported exceptional results for menstrual pain, to publish a clinical study and obtain US market clearance, we are collaborating with the University Hospital at Birmingham, UK. The researchers here are world renowned thought leaders in women's health and are conducting a double-blind randomized controlled trial to evaluate the efficacy of ActiPatch in reducing menstrual pain (clinicaltrials.gov listing NCT03394547). You can view the existing clinical evidence and our commercials at https://www.myallay.com/ Prevention of Episodic Migraines Headaches Migraines affect 36 million men, women and children in the United States alone. The facts are:
Chronic pain is now widely understood to be due to central sensitization, which leads to exaggerated pain perception. Migraine is no exception; since it is well known that sensitization of the trigeminovascular pain pathway can occur during a migraine attack. There is pilot data that ActiPatch can help mitigate this sensitization, so a study has been completed to determine the efficacy of ActiPatch in preventing chronic, episodic migraines. The data from the study will be analysed in the next few weeks. We believe this data will allow us to work towards developing a product as a migraine therapy and allowing us to obtain market clearance. Postoperative Recovery and Pain Working with our distributor in Lebanon we are conducting a double blind placebo randomized controlled trial on total knee replacement (clinicaltrial.gov NCT03395444). Through our long standing association with B. Braun UK, RecoveryRx has been determined to be a valuable aid in joint replacement surgery. This clinical study will allow us to gain worldwide recognition and allow for expanded marketing and acceptance of the RecoveryRx as a standard of post-operative care following joint replacement. Interstitial Cystitis (Overactive Bladder and Pelvic Pain) This study is a University of Texas, McGovern Medical School sponsored double blind randomized controlled trial. The goal is to determine how well the ActiPatch therapy works in treating patients with interstitial cystitis, bladder pain syndrome and overactive bladder. Interstitial cystitis and bladder pain syndrome are chronic bladder health conditions that greatly affect quality of life. These conditions create intermittent feelings of pain and pressure in the bladder area. The study is expected to recruit 60 women who are urology patients of the University's hospital. At least 35 million Americans have overactive bladder. Lower urinary tract symptoms, urgency, and pelvic pain are common complaints to urologists and primary care physicians. Additional Bioelectronic Product Opportunities As we develop the organizational structure we envision additional opportunities in chronic wounds, neuropathy, hypertensive therapy, etc. Most importantly future growth is not dependent on large capital outlays for research and development. Immediate Sales Growth We anticipate imminent solid sales growth from the following programs:
Thank you for your support. Sincerely,
______________________ |
FREDERICK, MD., May 31, 2018 (GLOBE NEWSWIRE) — BioElectronics Corporation (OTC PINK: BIEL), maker of advanced medical devices announces that B. Braun Ltd, of Sheffield, UK has recently completed development of its same day surgical TOTAL Pathway program for joint replacements which includes BioElectronics medical devices. The program is being launched by B. Braun’s UK Group to improve care and reduce the UK healthcare cost of its 160,000 annual hip and knee replacement procedures.
The “fast-track” same day hip and knee replacements are being supported by hospital physiotherapy teams visiting the patient at home and communicating using a specialized wireless tablet. Each patient will be given an ActiPatch medical device and a prescription for 6 additional ActiPatch devices. A similar program is being implemented for spinal surgeries.
Phil Cleary, Senior Product Group Manager, stated that “our TOTAL Pathway program enhances our commitment to patient safety, post-operative care, as well as significantly reducing healthcare costs and hospital stays.” Treating post-surgical pain requires a multifaceted approach. In trials, ActiPatch was well received and produced positive outcomes. We look forward to making this program a success, in the UK and other countries where B. Braun operates.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
About B. Braun Medical Ltd
B. Braun Medical Ltd, UK, https://www.bbraun.co.uk/ is a member of the B. Braun Group, https://www.bbraun.com/en.html one of the world´s leading healthcare companies. On an international level, B. Braun’s 54,000 employees are in 60 countries and are separated into additional divisions for hospital care, surgical products, outpatient care, and home care services.

Keep in mind partners are happening and licensed ones at that.
The back study will not go in as a new submission, it is included in the knee and ankle submission so this will happen quicker than most would believe. Well actually the back study will be added to the prior submission, I don't want to confuse you
Watch how huge the Co-Branding gets. How many names besides Pain Gear?
Increasing shareholder value=7 components of shareholder value.
• Revenue
• Operating Margin
• Cash Tax Rate
• Incremental Capital Expenditure
• Investment in Working Capital
• Cost of Capital
• Competitive Advantage Period
Within the next 120 days BIEL will have in place-
Full body clearance minus the Migraine, anticipated clearance at a later date based upon the fact that this is a preventative study and the patients must have signs of a migraine coming, this study is a little more complicated.
Licensed partners (Major)
Equity partners
At least 2 huge retail chains
Let’s add on a few more distributors. Why not? Lol
NHS-first week of April, keep in mind not the only week, this is lifetime!
There will be other EU States that will duplicate the NHS and this will be coupled with licenses being sold to partners. What price, how much in royalties will soon be determined.
The August financials will and should be the strength of evidence that a certain person has established a supply chain that will be second to none.
I should mention that sales makeup only one component of the revenue stream, this is where my opinion may differ from the good posters on the BIEL board.
Revenue is the total amount taken in by a business in a set period of time.
This can include royalties, license fees and equity from partners and sales.
BIEL cannot have millions in sales without including the other revenue streams mentioned above.
My revenue forecast including sales may be different than others.
Great rewards will follow in due time. Waiting on the FDA protocol. Which means pre-submission. Guidance, rules and timing will be part of the protocol.(Looks like May-June clearance.)
Migraine clinical results will still be the huge one for the FDA protocol.(August-September clearance on positive results.)
NHS is the first of many EU States getting on board. Pretty good health systems across the pond. Sweden next!
The back pain study will obtain US FDA clearance for relief of musculoskeletal pain. Migraine headaches are not musculoskeletal. Migraines and musculoskeletal pain are both neurological disorders. Two FDA clearances!!!!!!!!!!!!!
SEC-much ado about nothing!
Partners-much ado about something!
12/7/2017 - Try and Tell US Launch, gets a 7 Day ActiPatch to US residents for shipping and handling costs of $4.95
12/7/17 - BIEL closes a deal with ANDA for National Distribution, ANDA has 60,000 commercial customers and is owned by TEVA Pharma, TEVA has extensive business dealings with the NHS
1/4/2018 - ActiPatch gains admission to the UK NHS Drug Tariff as a covered medical device, The NHS is the largest single healthcare delivery organization in the world
1/9/2018 - CARE Pharmacies will be handling the ActiPatch, CARE is an organization of 90 pharmacies in 17 states, CARE will be educating patients on the benefits of drug-free pain relief through Pain Consultations
1/16/2018 - Allay Menstrual Clinical Study is fully enrolled, taking place at the prestigious Birmingham Women's Hospital,an NHS Foundation Hospital
1/22/2018 - BIEL signs deal with Performance Health, 120 outside sales reps, 40 inside reps, KN interview
1/22/2018 - BIEL signs deal with MundiPharma in Australia and 8 other countries, Mundi is associated with Purdue Pharma, KN interview
1/22/2018 - Back Pain Study is complete, BIEL has dialogue ongoing with FDA for submission of Study to expand ActiPatch indications, KN interview
1/22/2018 - Migraine Study will get the full attention of staff now that the Back Study has been completed
1/22/2018 - BIEL in talks with Walmart
1/22/2018 - Meeting scheduled with CVS
This is the last 2 months of activity for BIEL. Any other OTC company would be dancing in the Street if they had accomplished this much in the last year.
The NHS Approval has opened new doors for BIEL and will continue to do so for many years.

FREDERICK, Md., Jan. 04, 2018 (GLOBE NEWSWIRE) -- BioElectronics Corporation (OTC PINK: BIEL), www.bielcorp.com makes wearable, drug-free chronic pain therapy medical devices. It is pleased to announce that the UK’s government-funded public health service, National Health System (NHS), has approved our application to cover and pay for ActiPatch® Musculoskeletal Pain Therapy. The NHS estimates that almost half of the 50 million UK adults may be living with chronic-pain¹, which is why there are more than 100,000 physician visits every day for musculoskeletal pain alone².
The ActiPatch is a drug-free, wearable medical device that regulates peripheral nerve activity to provide pain relief. The NHS based its decision considering strong clinical evidence and a health economics study, which found that ActiPatch significantly decreased pain and improved quality of life, while reducing overall healthcare costs by 42% (58.5% reduction in physician appointment costs, 35% reduction in prescription medication costs).
“This is a major win for pain sufferers in the UK, since they will now be able to obtain a prescription for ActiPatch, the cost of which will be covered by the government,” stated Ian Rawe, Ph.D., Director of Clinical Research at BioElectronics. “We commend this move by the NHS, as this will open up the doors for reimbursement in the US and other managed care markets. This will likely have a tremendous impact on our sales and marketability of the product in the UK and elsewhere,” said Keith Nalepka, VP of Sales & Marketing at BioElectronics.
With the addition of ActiPatch to list of approved treatments, chronic pain sufferers now have access to paid safe, drug-free chronic pain relief. The ActiPatch will be listed for payment coverage in April.
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
References
1 NHS, "NHS Choices," January 2018. [Online].http://www.actipatch.com/actipatch/wp-content/uploads/2014/11/ActiPatch-3-month-Cost-Study-Final.pdf
Available: https://www.nhs.uk/news/medical-practice/almost-half-of-all-uk-adults-may-be-living-with-chronic-pai....
2 Arthritis Research UK, "Muscloskeletal Matters," Keele University, 2009
ActiPatch Cost Assessment Study Used By UK's NHS
http://www.actipatch.com/actipatch/wp-content/uploads/2014/11/ActiPatch-3-month-Cost-Study-Final.pdf
This improvement translated into reduced utilization of healthcare services, leading to a 36.8% reduction in health care costs to the NHS over a 3-month period, even after accounting for the cost of the ActiPatch device
Conclusion
Utilizing ActiPatch® as a chronic pain therapy treatment improves patient quality of life, while reducing the economic burden to the NHS.
"Almost half the adult population is living with chronic pain," the Daily Mail reports. A major new review suggests that around 28 million adults in the UK are affected by some type of chronic pain (pain that lasts for more than three months). Read More Link
https://www.nhs.uk/news/medical-practice/almost-half-of-all-uk-adults-may-be-living-with-chronic-pain/
February 06, 2017 09:05 ET
FREDERICK, MD--(Marketwired - Feb 6, 2017) - BioElectronics Corporation (OTC PINK: BIEL), the maker of wearable pain therapy devices, announced today that it has received over-the-counter use market clearance from the US FDA for ActiPatch® for the adjunctive treatment of musculoskeletal pain related to (1) plantar fasciitis of the heel; and (2) osteoarthritis of the knee.
BioElectronics is an electroceutical company that develops wearable, neuromodulation devices to safely mitigate neurological diseases and improve quality of life. Our innovative pulsed shortwave therapy technology (PSWT) that uses low power pulsed electromagnetic fields regulate electrical activity of the nervous system. The neuromodulation basis of PSWT presents significant opportunities for BioElectronics to develop optimized technology for diabetic neuropathy, postoperative surgery, chronic wounds, and other applications.
Our current OTC product line includes ActiPatch® Musculoskeletal Pain Therapy, Allay® Menstrual Pain Therapy, Smart Insole™ Heel Pain Therapy, and RecoveryRx® Post-operative and Chronic Wounds Therapy. The US FDA clearance is for our flagship product the ActiPatch® Musculoskeletal Pain Therapy, developed to relieve chronic pain. ActiPatch is a drug-free, wearable nonprescription medical device that provides 720-hours (90, 8-hour treatments) of on/off therapy for $30.00 retail. Most users obtain relief with only 8 hours per day of use, so the device will generally last several months, depending on use. ActiPatch Provides:
About BioElectronics Corporation
BioElectronics Corporation is a leader in non-invasive electroceuticals and the maker of an industry leading family of disposable, drug-free, pain therapy devices: For more information, please visit www.bielcorp.com.
How to Order an ActiPatch®?
Visit tryactipatch.com to place your order for the ActiPatch® today.
www.tryactipatch.com
BioElectronics Corporation
301-874-4980
info@bielcorp.com
4539 Metropolitan Court
Frederick, MD 21704
ActiPatch Healthcare Utilization Study Link
http://www.harmonyhealth.se/wp-content/uploads/2017/10/ActiPatchHealthcareCostStudy.pdf
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |