Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
just look what Gerald Commissiong - twice failed, disgraced, flunkey loser AMBS CEO ...
did to his faithful TOMDF shareholders in a mere few years time:
Printing $0.000001 - and now permanently dissolved:

what a pathetic excuse of a disgraced CEO who scammed shareholders in 2 public securities

IMHO, this guy should investigated & in jail
along with his Astoria, Queens, financial mafia co-conspirators...
TOMDF lost $138-Mil. in just under 5 years time - where did all the money go Gerald...?
inquiring minds want to know...!
IMHO, this scam artist will never work on Wall St - ever again...
Managing Partner at Fortitude Advisors
https://app.qwoted.com/companies/fortitude-advisors
can't these guys would have Gerald associated with their name
https://fortitudeadvisors.com/
AJMHO
Yeah… sickening if this one doesn’t come back.
32 AMBS CEO Blogs by Gerald Commissiong, CEO

and not one blog ever had "any credibility" whats-so-ever to it - just all lies and fabricated realities...!
and they were permanently removed from the internet - what's that tell you...?
http://www.thechairmansblog.com/amarantus-bioscience/blogs/
IMHO, this guy is a fraud and a huckster - always was - always will be...!
AJMHO
I sold my remaining 2 AMBS shares on Friday. I made a profit on my previous sale of 600K shares, but took a small loss on this sale. 😎
I was only keeping these 2 shares so I could post here as a shareholder and empathize a bit with those with the catastrophic losses on their six-figure investment. The ones who are still posting here and still holding their shares a decade later—clearly an expression of their faith and confidence and continued hope for the ultimate success of their Amarantus Biosciences investment. One has to wonder if they're still having private phone calls with the CEO.

https://investorshub.advfn.com/boards/read_msg.aspx?message_id=167164997
https://investorshub.advfn.com/boards/read_msg.aspx?message_id=168016251
It's a good thing you're still holding all of your AMBS shares, as you've posted numerous time. You'll be ready when something develops.
I sold my investment in GC's other company and rolled proceeds into another investment, where I'm already up six figures.
Thanks GC for pointing me in that direction.
dead - simply impossible...!
not with all of these AMBS CEO Blog promised developments in the werks:
Amarantus Bioscience – Chairman’s Blog
http://www.thechairmansblog.com/amarantus-bioscience/blogs/

you mean they were nothing more than Gerald Commissiong's bold-faced lies & self-fabrications...?
SMH folks - twice failed AMBS & TOMDF CEO stooge for the Astoria Queen's financial mafia
they walk away with $-Millions while Gerald the patsy holds the proverbial Wall St indelible stain rep...!

Ask yourself this: was it all really worth scamming the public out of their investment dollars...?
SMH - sad but true
MOO lick spiddles & toties
Clearly there are issues for you reading a statement and understanding it. You keep highlighting the same text that doesn't answer the question posed.
Where exactly in my post do I say anything about fake IP address, burner phones, or multiple aliases. The only thing mentioned is fake credentials (name and email address) and I stated reason why.
The rest of it you're pulling out of your ASS.
That's it. The last one. You can't provide an accurate or intelligent answer, so you keep throwing the same turd at the wall to see if it will stick. Kinda like how you make investment choices. 💩 💩 💩 None sticking for last 10 years. No zip code change for you.

here you go Joey-the-Forks-Bolo
served up to you ...
just like room service Sporto

uh oh, Gerald's "WeWorks" cubicle ...
must have gotten emptied out in a hurry - eh JOEY...?

but hey, at least you got some toilet paper left
to clean up the mess he left you...!
SMH - can't make this stuff up folks
MOO bad brains
Where exactly in my post do I say anything about fake IP address, burner phones, or multiple aliases. The only thing mentioned is fake credentials (name and email address) and I stated reason why.
The rest of it you're pulling out of your ass. And you have no clue about VPNs and IP addresses. Did your crack IT team at Dominos ever discover who was sending you pizzas? Why haven't you updated us on that?
MiamiMark (whoever the f*ck that is)... how is that relevant? Your new infatuation, perhaps? Kicked GC to the curb? Maybe it was this guy sending the pizzas?

Man Behind the Buzz
Gerald Commissiong - twice failed Stanford University "Financial Engineer" CEO hack
now looking for your money - so he can do it a 3rd time
my-o-my ... how just a few years can change things on a NY dime
especially when things are built on illusions, deceiving SH's and a Make It Up as We Go Along portfolio

SMH - only in America can you rip people off and get away with it
all that's left are broke AMBS and TOMDF SH's, and an indelible 'stain' on Wall St.
MOO sad
how's that "MANF March" doing Gerald...?
Amarantus Bioscience – Chairman’s Blog
http://www.thechairmansblog.com/amarantus-bioscience/blogs/
you know the one - AMBS CEO Blog #29

29.) MANF Begins its March Towards the Clinic
May 14th, 2015 - by Joseph Rubinfeld
Throughout my 50+ years in the biopharmaceutical industry, I have seen many programs with great promise, but very few programs that possess the fundamentally groundbreaking scientific potential to modify disease biology inherent in MANF. In the early days of Amgen, I remember EPO was Number 9 on the priority list for product development. I had to lobby extremely hard to get it moved up the ranks to Number 5 (largely due to the concept of using it in an orphan indication, replacement therapy in kidney dialysis patients). Despite the strong science behind EPO, most people in Amgen’s leadership wanted to focus on some other perceived near term potential opportunities like interlukins, inteferon, chicken growth hormone, malaria, hybridomas, etc. Without this lobbying, I am not certain we would have seen the Amgen we now know today.
I saw a similar situation with Amarantus in 2012. Towards the end of that year when I first began investing in Amarantus, and initially joined the Company as an advisor, I believed that the vast potential of the MANF program could be harnessed into a major medical breakthrough by expanding the focus of preclinical studies beyond Parkinson’s disease, and into a broader area of orphan drugs. In 2013, the Company had its first breakthrough in this area, through some groundbreaking work from the University of Miami’s Bascom Palmer Eye Institute. By demonstrating that MANF had tremendous potential to protect rods and cones in degenerative models of retinitis pigmentosa (RP), we had identified an orphan indication for which no competition existed in the market that would allow the Company to conduct some additional proof of concept work to validate prior to moving MANF into the clinic. Shortly thereafter, we generated data supporting the use of MANF in a broad range of ocular conditions, including orphan conditions such as retinal artery occlusion, as well as large indications such as glaucoma. Taken together, MANF’s potential to treat vision loss associated with a range of ophthalmological conditions alone makes it a compelling product opportunity worthy of further development.
However, we now know full well ophthalmology is just the beginning of the MANF opportunity. Either by internal development, collaboration with academics, or evaluation of the scientific literature, we now know that the MANF program’s potential expands far beyond ophthalmology, and into the areas neurology, otology, cardiology and metabolism (diabetes), among others. This vast potential for MANF is what makes today’s announcement personally so exciting for me, as the initiation of IND-enabling studies (which start with initiation of clinical grade manufacturing) has been the rate-limiting step for true product development value creation for MANF not only for the ophthalmology programs, but also the rest of the MANF pipeline. We have spent the last 12 months evaluating clinical grade material suppliers in parallel with generating the scientific and regulatory framework to support a robust IND-enabling program that will take MANF into the clinic where we can get a first glimpse of MANF’s human efficacy profile. We believe Catalent is the best clinical-grade supplier to support our development, and are extremely pleased that the process is now moving forward. Before this major step forward MANF was a wish, now it is on the path to becoming a reality.
Going forward, we will be formalizing agreements with key constituents including key opinion leaders, patient advocacy groups and regulatory experts in order to drive this program into the clinic and begin to treat patients suffering from the many afflictions MANF may impact. This is where true value will be generated and we are extremely pleased that the process has now begun.
As a Board member, I am very pleased to be helping guide the Company’s strategy forward as we have created a truly de-risked product development pipeline that can create value in many different areas, all the while providing MANF with the necessary infrastructure to be diligently moved forward without the need to rush its development and compromise its true value.
Thank you for your continued interest and support, we look forward to sharing more updates as MANF progresses towards the clinic.
Best,
Joseph Rubinfeld, PhD
Independent Director
Amarantus BioScience Holdings
I don't know velcro...
Fortitude Advisors' still carrying GC as NY managing partner - only thing I can think of is Fortitude maybe doing a toxic deal and may need the exposure to the Astoria Queens financial mafia
either way looks like GC was permanently 'hung out to dry' by TOMDF hierarchy, including JF and the back office admin... which makes sense - those white collar boys make hefty coin on the toxic convertibles and maybe from a few offshore havens
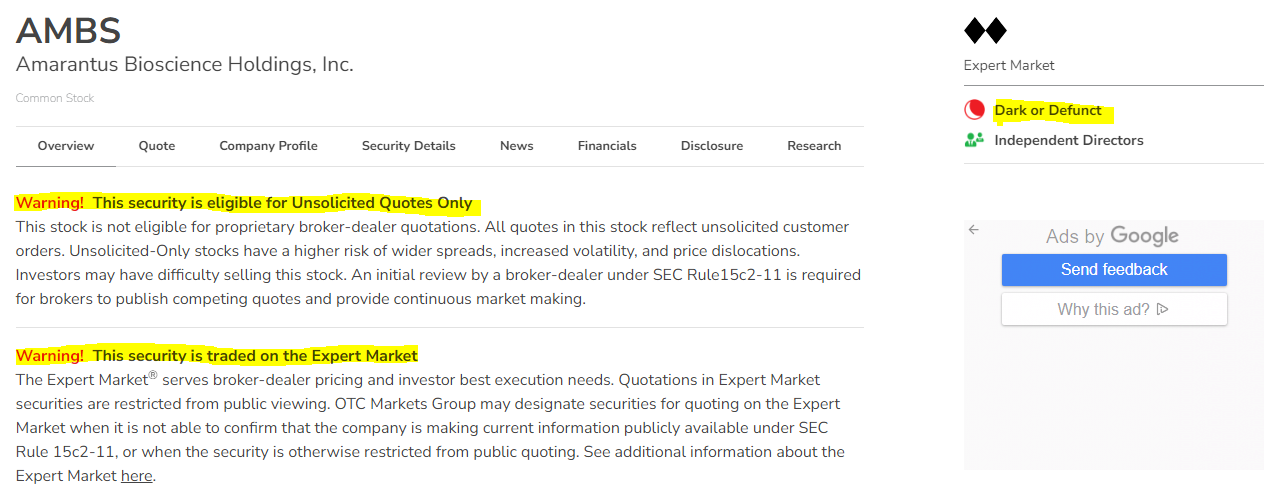
what we do know is TOMDF is 100% dissolved while AMBS is OTC default, dbl. black diamond expert market and non-reporting - might as well be declared BK
AJMHO
Fortitude Advisors did not need a clown on their staff.
glad Fortitude Advisors chose not to sully their reputation
with that of a 2 time looser sham con-artist serial toxic death spiral diluter
and flunky Stanford University “Financial Engineer” fake CEO hack…!
AJMHO
Gerald Commission no longer appears on the Fortitude Advisors website www.fortitudeadvisors.com
Obviously he was a negative drag on their credibility.
AMBS TA Chart & CEO Update
AMBS on a run-away southbound out-of-control pps TRAIN WRECK these past few years...!
all under Gerald Commissiong - twice failed public securities CEO hack
and Stanford University "Financial Engineer" patented flunky

got to love the fact that Gerald Commissiong TOMDF terminated CEO has permanently moved on...
from the "DISSOLVED & OUT OF BUSINESS" TOMDF right quick
via the Israeli insolvency judicial system - in only +5 weeks time - Wowza...!
Todos Medical Announces Insolvency Proceedings in Israel
December 22, 2023 17:28 ET
https://www.globenewswire.com/en/news-release/2023/12/22/2800853/0/en/Todos-Medical-Announces-Insolvency-Proceedings-in-Israel.html
and just LOOKIE HERE - now a Managing Partner with Fortitude Advisors in Austin Tx:
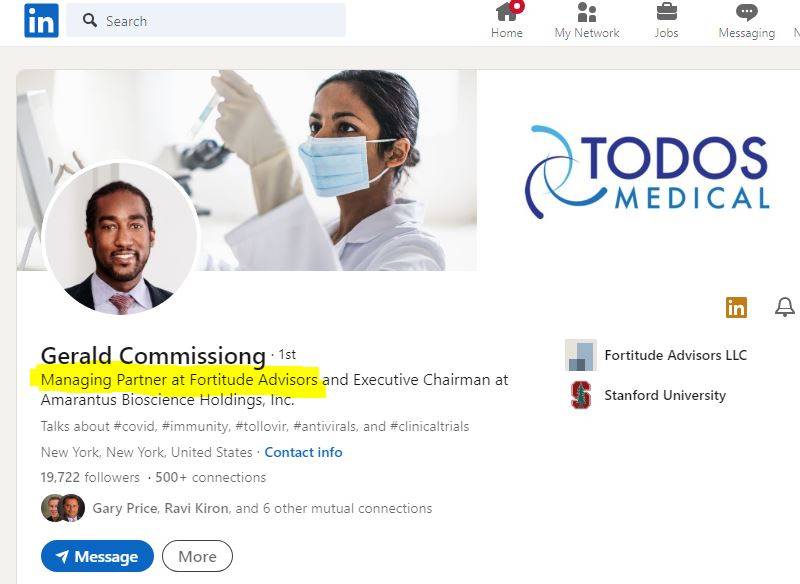
even sporting a new PhD - or is that a mistake for PnD...?
if you can believe this folks - here it is - in live and living color no less:

Website
https://fortitudeadvisors.com/
Phone
512-664-2050
well Sham Wow Gerald





Psalm 23:5: "You prepare a table before me in the presence of my ENEMIES. You anoint my head with oil; my cup overflows. Surely goodness and mercy will follow me all the days of my life, and I will dwell in the house of the LORD forever.…"
sorry to say - Gerald Commissiong's been a bad man...!
just CLICKIE HERE to see that AMBS's other kissing-cousin scam TOMDF is now totally dissolved - and rightfully so:
https://todosmedical.com/about
***** BANKRUPT - INSOLVENT - LIQUIDATED - DISSOLVED - TERMINATED - OUT OF BUSINESS - CLOSED *****
got to applaud the efforts of TOMDF's Founder & former Dir., Pres. & CEO - Rami Zigdon in shutting the TOLLOVID SCAM down
Todos Medical Announces Insolvency Proceedings in Israel
December 22, 2023
https://finance.yahoo.com/news/todos-medical-announces-insolvency-proceedings-222800017.html
only took the Israeli Judge 6 weeks to put TOMDF scam to "FINAL REST"
and to exemplify Gerald Commissiong as a disgraced, twice failed CEO hack - who'll now be hung out to dry
would you or your family trust this man with your money - ever again...?
aprox. $138-Mil. trhough the TOMDF books in 5 years - what happened to all this money...?
***** INVESTIGATE GERALD COMMISSIONG - TOMDF CEO *****

"You prepare a table before me in the presence of my enemies. You anoint my head with oil; my cup overflows. Surely goodness and mercy will follow me all the days of my life, and I will dwell in the house of the LORD forever.…"
oh yeah, what's the volume...?
OEM
What is our status here? Nice buy today...why? TIA for any info....
How stupid can you be what a garbage
Todos Medical Announces Insolvency Proceedings in Israel
Press Release | 12/22/2023
https://www.wallstreet-online.de/nachricht/17658569-todos-medical-announces-insolvency-proceedings-israel
WOWZA - Tollovid is now "burnt offerings" - simultaneously TOMDF's "books are cooked"
TOMDF will soon be BK, as it teeters on the proverbial edge of unreconcilable insolvency
also with the twice failed Stanford "Financial Engineer" CEO hack Gerald Commissiong being the quintessential "link" between TOMDF and AMBS...
it stands to reason that TOMDF going BK will have a significant adverse impact on AMBS
not to believe so would only enforce ones own folly and inevitable downfall

would you trust this man with your family's hard earned honest money...?
MOO sad
Some action today
yeah right - like were going to believe you ...
with your multiple aliases, burner phones, and fake IP addresses...?
ain't that right JOEY - or is it Waprcore - lolzzz...!
so many aliases - so much pumpin to do - your credibility's now permanently shot pal....!

SMH - get back on dem phones...!
MOO tout
Let's start with a penny. That's 10x from here and realistic in the near term given some of the catalysts re Magna, MANF, and 3CL. That would have the company at a $6.3M market cap. I mean why not? And why not a dime from there if the skies start clearing?
typical childish answer.
well let me refresh your memory. Wasn't it you that criticized a poster for making a post and not owning any shares? Memory gone Joey? Guess you must have forgotten to take your meds this morning. Since GM posted on this board we assumed he was a shareholder of AMBS. Guess your buddies are OK doing that. And while we are discussing posting on a board without being a share holder I can only assume you still own shares in AMBS. Unless you are still a hypocrite and GC ass kisser.
Don't forget your meds Joey. Wink, wink
You're not making any sense. You must have contracted Covid multiple times like JP and it's affecting your ability to think or remember things. GM never invested in AMBS. He did invest in Todos and still owns those shares, as I do. Nothing else in your post makes any sense.
But believe whatever you want. JP can help you with recommendations for 9 specialists for your deteriorating cognitive ability. Don't forget to take your Geritol daily. And your Metamucil for regular bowel movement.
one grade ahead of you hypocrite. Guess he must have just sold as his post was resent. See your're still around haven't sold yet Joey?
one grade ahead of you hypocrite. Guess he must have just sold as his post was resent. See your're still around haven't sold yet Joey?
Had GM ever invested in AMBS, he would at least have been man enough to sell and move on by this point, and not spend a decade crying on a public message board about it. And if he believed the CEO was a scammer, he certainly wouldn't have been dumb enough to invest with him again. That decision was YOURS. You can't blame that on the CEO.
Just the facts, Sherlock!
"Looky here"? What, are you stuck in 6th grade?
Well looky here at the hypocrite. Weren't you the one that took posters to task for posting when not being a shareholder? And by the way ABMS still trades shares. Doesn't have to be a shareholder from years ago. Could have bought on your consistent praise of GC. Look in the mirror and ask where your investment is now.
Only positive is AMBS is trading at twice the price of tomdf. What a freekin joke and ambs has no revenue. Let's see how you spin this one.
Just facts sherlock!
Great detective work, Sherlock. GM was still a struggling college student during AMBS days with no money to invest. Try again.
well looky looky who's on ambs board. Guess Greesy also was conned and lost money over here also. Welcome aboard.
That would remarkable
|
Followers
|
647
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
130513
|
|
Created
|
07/14/11
|
Type
|
Free
|
| Moderators | |||


| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |