Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
It looks like IVillage is online again but won't allow sign in with old passwords, anybody have any insight/opinion on what to do to best protect ourselves in this situation? Has anyone gotten any correspondence from them about the situation?
I agree, the current SP is down right silly, and when this takes off it will be amazing for all long term suffering investors lol
When you read thru the last two posts on this board and the latest Provectus Substack posting, you can not help being impressed with the potential of this company. Combine that with the `shareholder value' theme that Dom and Ed constantly align themselves with, I just can not see why we are trading for a couple dimes per share. Collaborations with Moffet Cancer, Univ of Miami/Bascom Palmer, Univ of Calgary, Univ of TN, HD Anderson and others sure think we are worth their time and effort. It feel like something will break in our favor soon. Yes much of this is all pre-trial, but progress is being made.
Identification and In Vivo Validation of Unique Anti-Oncogenic Mechanisms Involving Protein Kinase Signaling and Autophagy Mediated by the Investigational Agent PV-10
Simple Summary
Novel therapeutics are urgently needed for high-risk and refractory solid tumors. Clinical studies have demonstrated the safety and efficacy of PV-10 (10% rose bengal sodium) by intralesional injection in skin cancer. However, this agent has not yet been evaluated for the treatment of various adult solid tumors. The aim of our study was to test PV-10 in breast, colorectal, head and neck, and testicular cancers. Using a combination of in vitro and in vivo experiments, we found that PV-10 exhibits anti-cancer activity against a panel of human cell lines derived from these tumors. Our results support further clinical development of PV-10 for the treatment of solid tumors in adults.
link to webpage with paper
NEWS -- Provectus Biopharmaceuticals Announces Publication of Preclinical Data of Oral Administration of Provectus Rose Bengal for Solid Tumor Cancers
KNOXVILLE, TN, April 18, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that data from preclinical research by the University of Calgary on oral administration of Provectus’s pharmaceutical-grade rose bengal for the treatments of solid tumor cancers were published in the open access journal of oncology Cancers.
Titled Identification and In Vivo Validation of Unique Anti-Oncogenic Mechanisms Involving Protein Kinase Signaling and Autophagy Mediated by the Investigational Agent PV-10, a copy of the University of Calgary’s journal article is available here: https://www.mdpi.com/2072-6694/16/8/1520.
This work has been part of research conducted by Aru Narendran, M.D., Ph.D., professor in the departments of Pediatrics, Oncology, and Biochemistry & Molecular Biology, and members of the Narendran Lab at the University of Calgary’s Cumming School of Medicine in Calgary, Alberta, Canada.
The Narendran Lab provided preclinical proof-of-concept data supporting the efficacy of Provectus’s rose bengal in a panel of adult solid tumors. The Lab identified that the Company’s rose bengal downregulated WNK1 and Wnt signaling. In mice, the Narendran Lab also confirmed the clinical utility of Provectus’s rose bengal by intratumoral (aka intralesional) administration and demonstrated potential utility by oral administration.
Dominic Rodrigues, the Company’s President and Vice Chairman of its Board of Directors stated, “Provectus is focused on initiating an FDA-cleared, lead clinical development program for intratumoral cancer immunotherapy PV-10 to treat hepatic metastatic pancreatic cancer, and pursuing a path to an initial drug approval for PV-10. The Company previously demonstrated its systemic response and systemic immune signaling and activation in clinical settings for metastatic liver cancers.”
Mr. Rodrigues added, “Building on PV-10’s clinical safety, efficacy, and immunotherapeutic profile, the Narendran Lab’s preclinical data suggest that Provectus’s pharmaceutical-grade rose bengal delivered by oral administration may have the potential to treat a larger pool of solid tumor cancers.”
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information provided in this press release may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission, including those described in Item 1A of:
The Company’s Annual Report on Form 10-K for the period ended December 31, 2023.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
LTG, sure hope you're right about a rocket to da moon soon. And glad to see some IVillagers posting here, let's use this site until IV can recover their site and once again provide a safe posting place for us. I'm not a facebooker but my wife is and asked to join that group, but we see no posts there today so we wonder how active it is anyway. Not that IVillage was all that active, outside of BM who I've generally kept on ignore the past few years!
My best to everybody in PVCT land!!
For all the PVCT people that can not get into IV. We would love to have you join us on our PCVT Facebook chat if you are interested.
Thanks for the advice, getting any IV info is sketchy. Hope you and Wehalls keep posting here. Eye company organization should be disclosed soon, will be nice to have that $ commitment known . . . and behind us. A revenue stream in 12 - 16 months would be awesome.
That site is all the way hacked. I doubt the owners have any control over the site as of now.
I would HIGHLY recommend NOT giving them any payment information and I wouldn't even log into the site using a Username and Password that most have across numerous websites.
We can all assume our UID and passwords have been compromised. However, I believe the payments we've sent to the site IN THE PAST are still secured because it was through a 3rd party. Do NOT PUT NEW PAYMENT INTO INTO THE SITE. I also believe IV owes us all some money for being offline for so long.
Also, PVCT is about to rocket to the moon!!!!!
NEWS -- Provectus Biopharmaceuticals Announces Management Additions and Reiterates Commitment to Shareholder Value Creation
KNOXVILLE, TN, April 16, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced additions to its executive leadership team, reflecting Provectus's dedication to enhancing shareholder value. The Company’s Board of Directors (the “Board”) has appointed Ed Pershing as Chief Executive Officer (“CEO”) and Dominic Rodrigues as President.
New Executive Appointments
Mr. Pershing has served as Board chairman since 2018 and was a Board observer and chairman of the Company’s Strategic Advisory Board from 2017 to 2018. Mr. Rodrigues has served as Provectus’s chief operating consultant in 2024 and vice chairman since 2018 and was chairman from 2017 to 2018. They will continue to serve in their respective Board roles.
Mr. Pershing co-founded Pershing Yoakley & Associates (“PYA”) in 1983 and was its President and CEO until his retirement from the firm in 2019. PYA is a top 20 healthcare consulting and top 100 accounting firm in the U.S., growing from a three-employee office to more than 350 employees and four affiliate companies serving more than 3,500 clients in all 50 states. Mr. Pershing’s healthcare experience and expertise include turnaround and performance improvement initiatives, long-range planning studies, development of numerous hospital and medical office projects, restructuring of healthcare organizations, liaison between boards of directors and management, mergers, acquisitions, divestitures, and leasing arrangements. He also served as an expert witness on healthcare industry matters and represented healthcare organizations before federal and state regulatory agencies.
Mr. Rodrigues worked in management consulting and corporate development in science and technology-driven industries prior to Provectus. He also was a finance professor at the University of Nevada, Las Vegas, a venture capitalist at defense contractor SAIC, a currency derivatives trader at Bank of Montreal, and a project manager and engineer at Jacques Whitford. Mr. Rodrigues holds business, economics, engineering, and public policy degrees from The Wharton School, the London School of Economics, MIT, and the University of Toronto.
Mr. Pershing stated, “Since 2017, Mr. Rodrigues has managed clinical development, research collaborations, key vendors, and other Provectus activities in all biotechnology business functions, supported our finance and accounting team, and been a contributing member of the board of directors.”
Mr. Pershing added, “Mr. Rodrigues has secured existing molecular biochemical, medical scientific, and manufacturing process knowledge from Provectus’s former founders and contributed to the creation of new insights in these areas. His efforts ensure that all corporate understandings can be efficiently conveyed to new team members and development partners in the future.”
Mr. Pershing concluded, “Mr. Rodrigues and I first met as fellow shareholders at the 2010 annual meeting of the American Society of Clinical Oncology where Provectus clinical data were being presented. We have long shared a supreme confidence in the singular distinctiveness of the Company’s rose bengal sodium molecule. In asking him to join me in 2017 to save Provectus and its truly unique science, Mr. Rodrigues unequivocally embraced my vision of pushing the molecule to achieve its true potential as an expansive therapeutic platform capable of transforming the way the healthcare industry treats disease. With our team of dedicated, perseverant executives, employees, consultants, and clinical and research collaborators, we will continue to execute on our mission to develop and commercialize innovative, broad-spectrum, immunotherapy medicines that are safe, effective, accessible, and affordable, and that can revolutionize the healthcare industry as we know it.”
Commitment to Shareholder Value Creation
Provectus remains committed to delivering long-term value to its shareholders. On the Company’s First Quarter 2024 Conference Call, Provectus outlined its key areas of focus for 2024:
I purchased the lifetime membership several years ago when Blue was trying to raise money, but I think nine dollars is a reasonable access fee and helps support the site. As they say, nothing is free.
I just looked at the website, and the free access is no longer available.
I keep getting a message that says no more ad supported free service. $9 a month to use now.
Yes they are back up now though I’m not sure about the pay membership only. He is rolling it out as he can.
From the conclusions on the Poster.
PV-10, an IL immunotherapeutic agent, demonstrates significant anti-tumor activity in
HNSCC.
• Our In vitro studies show PV-10 induces potent ICD in HNSCC cells.
• Our In vivo experiments show IL PV-10 lead to substantial tumor regression and complete
responses in some mice.
• These findings underscore the significance of PV-10-induced ICD which may offer a novel
and promising approach for managing HNSCC and potentially pave the way for improved
survival rates and reduced adverse events
NEWS -- Provectus Biopharmaceuticals Announces Presentation of Cancer Immunotherapy PV-10 Poster for HNSCC at AACR 2024 Annual Meeting
KNOXVILLE, TN, April 11, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that data from preclinical research by Moffitt Cancer Center (“Moffitt”) on PV-10 (rose bengal sodium) for the treatments of human papillomavirus (“HPV”)-positive and HPV-negative head and neck squamous cell carcinoma (“HNSCC”) were presented at the annual meeting of the American Association for Cancer Research (“AACR”), held April 5-10, 2024 in San Diego, California.
Titled PV-10 triggers immunogenic cell death and anti-tumor immunity in head and neck squamous cell carcinoma via endoplasmic reticulum stress and autophagy, a copy of Moffitt’s poster presentation is available on Provectus’s website at: https://www.provectusbio.com/media/docs/publications/AACR-poster-3-Apr-2024.pdf.
This work has been part of research conducted by Christine Chung, M.D., Chair, Department of Head and Neck-Endocrine Oncology and Program Leader of Head and Neck Oncology and members of the Chung laboratory at Moffitt in Tampa, Florida.
Moffitt observed that PV-10 induced reactive oxygen species-mediated apoptosis, surface expression of calreticulin, and extracellular ATP and HMGB1 release in HNSCC cells. PV-10-induced immunogenic cell death (“ICD”) led to surface expression of HSP-70 and HSP-90 in these cells. Intralesional (“IL”) injection of PV-10 promoted tumor regression in mice by inducing endoplasmic reticulum stress, triggering autophagy, and initiating apoptosis.
Moffitt concluded that PV-10 demonstrated significant anti-tumor activity in HNSCC. In vitro studies illustrated that PV-10 induced potent ICD in HNSCC cells, and in vivo experiments showed that IL PV-10 led to substantial tumor regression and complete responses in some mice. These findings, according to Moffitt, underscored the significance of PV-10-induced ICD, which may offer a novel and promising approach for managing HNSCC and potentially pave the way for improved survival rates and reduced adverse events.
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information provided in this press release may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission, including those described in Item 1A of:
The Company’s Annual Report on Form 10-K for the period ended December 31, 2023.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
Happy Easter!
Mark 16: 1-7
Resurrection Morning
16 When the Sabbath was over, Mary Magdalene, Mary the mother of James, and Salome bought spices, so they could go and anoint Him. 2 Very early in the morning, on the first day of the week, they went to the tomb at sunrise. 3 They were saying to one another, “Who will roll away the stone from the entrance to the tomb for us?” 4 Looking up, they observed that the stone—which was very large—had been rolled away. 5 When they entered the tomb, they saw a young man dressed in a long white robe sitting on the right side; they were amazed and alarmed.
6 “Don’t be alarmed,” he told them. “You are looking for Jesus the Nazarene, who was crucified. He has been resurrected! He is not here! See the place where they put Him. 7 But go, tell His disciples and Peter, ‘He is going ahead of you to Galilee; you will see Him there just as He told you.’ ”
The Holy Bible: Holman Christian standard version. (2009). (Mk 16:1–7). Nashville: Holman Bible Publishers.
I've owned CRMD in the past, I would have a lot of brushing up to do to get current. I do not feel very competent discussing the ongoing/current trials, others here are much more knowledgeable, but the Australian trial intrigues me the most. In the recent past,. there was talk about additional updates forthcoming from that trial and also the consideration of a Therapeutics Goods Administration (TGA - Australian version of the US FDA) PV-10 application, but have heard nothing since. Always felt like the Australian trial or UM ocular work would hit first. This morning's licensure agreement is positive but presently lacks the detail we need to forecast potential revenue flow or development costs for the new spinoff company; I'm sure further info will be available soon. Advancing any oncology trial to P3 status, therefore, is still a minimum of 1.5 years out from my take.
NEWS -- Provectus Biopharmaceuticals Announces Exclusive Worldwide License Agreement with University of Miami for Photodynamic Antimicrobial Treatment of Different Eye Infections with Rose Bengal Sodium
Been quite a while since I last owned shares (back in the 2017/18 timeframe). Been monitoring the developments from a big picture standpoint.
So after seeing the excitement over the Bascom PI activity, wondering if you have seen anyone project a 'timeline' on where things are vs when they might get to the end of a viable PH 3 for 'anything'..
Again, I am just ramping up my research into their status on their trials, et al.
Own no shares, but thinking committing $5000 at 20 cents or less would be a good idea.
Thanks
disclaimer: biggest position is in CRMD which is around $4, has recently been approved by the FDA and has a 'conservative' target price of $14. Sales are expected to commence before 1 July. Not a sexy development that attracts the market, but a NEEDED development. With a potential target price once they get approved for an even bigger sector of the market they could serve of around $25+ some... Yep, the potential for a very nice return, worth checking out..
I think we are beginning to see the light at the end of the tunnel!
PVCT has just improved from a lotto pick contender to long shot status with the Bascom Palmer Institute relationship (details yet to be announced). The Univ of Miami Bascom Palmer Institute agreement is the first step, of hopefully others, with far reaching clinical implications. IF the BPI agreement provides some much needed cash flow for the company in upcoming years (no licensure details as of yet), the oncological potential and timeline of PV-10 (PVCT's oncology development drug) could finally be elevated to the urgency and relevancy it so justly deserves. Present low n trials have produced substantial results for PV-10 but expensive trials of size/scope have long eluded us. Worse case, the BPI agreement at least legitimizes this company as a viable biotech partner.
BPI has said the new ophthalmological treatment using Rose Bengal has been ground breaking. Additional potential in oncology, wound/infection control, and even veterinary medicine lie ahead. Exciting times for this company thanks to PHR management who single handedly stabilized and refocused this once floundering company. Still a long way from profitability, plenty of hurdles remain ahead, odds remain against us, but finally there is a true light in the far distance.
BPI - Provectus
If my memory serves me correctly, they usually do provide the presentation they give at the conferences.
Any worthwhile presentation for the April 5-10 meeting?
NEWS -- Provectus Biopharmaceuticals Announces Notices of Allowance and Award for U.S. Patents of Rose Bengal Sodium in Virology, Pediatric Solid Tumor Cancers, and Vaccines
KNOXVILLE, TN, March 12, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that the United States Patent and Trademark Office (“USPTO”) has allowed patent application 17/212,723, titled “Novel Uses of Halogenated Xanthenes in Oncology and Virology.” The application covers the use of Provectus’s pharmaceutical-grade rose bengal sodium (“RBS”) drug substance for the treatment of infectious diseases, such as coronaviruses.
The USPTO has also allowed patent application 17/344,418, titled “In Vitro and Xenograft Anti-Tumor Activity of a Halogenated-Xanthene Against Refractory Pediatric Solid Tumors.” This prospective award covers the use of RBS in combination with one or more immune checkpoint inhibitors and is a continuation of U.S. patent 11,058,664 (2021), Provectus’s first for pediatric oncology.
The Company’s previously allowed patent application 17/488,430, titled “Halogenated Xanthenes as Vaccine Adjuvants” (December 2023) and covering RBS’s use as an adjuvant in vaccines to potentially make them work better, will be awarded on March 26, 2024 as U.S. patent 11,938,182.
Innovate Calgary, the innovation company of the University of Calgary in Alberta, Canada, is a co-assignee and Aru Narendran, MD, PhD, Professor of Pediatrics, Oncology, Biochemistry and Molecular Biology and Physiology and Pharmacology at the University’s Cumming School of Medicine is a co-inventor on all three patent awards.
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
The Company’s Annual Report on Form 10-K for the period ended December 31, 2022, and
Provectus’s Quarterly Report on Form 10-Q for the period ended September 30, 2023.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of Cancer Immunotherapy PV-10 Abstract for HNSCC at AACR 2024 Annual Meeting
KNOXVILLE, TN, March 07, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that data from preclinical research on PV-10 (rose bengal sodium) for the treatments of human papillomavirus (“HPV”)-positive and HPV-negative head and neck squamous cell carcinoma (“HNSCC”) will be presented at the upcoming annual meeting of the American Association for Cancer Research (“AACR”), to be held April 5-10, 2024 in San Diego, California.
The abstract, which was accepted for a poster presentation, is titled “PV 10 induces endoplasmic reticulum stress and autophagy, triggering immunogenic cell death and anti-tumor immunity in head and neck squamous cell carcinoma” (Abstract #6742, Topic Track: Immunology, Session: Vaccines, Antigens, and Antigen Presentation 2).
The work underlying this poster presentation is part of research conducted by Christine Chung, M.D., Chair, Department of Head and Neck-Endocrine Oncology and Program Leader of Head and Neck Oncology and members of the Chung laboratory at Moffitt Cancer Center (“Moffitt”) in Tampa, Florida.
According to Moffitt’s abstract, “…in vitro findings reveal that PV-10 induces cytotoxicity in both mEER and MTE-RAS cells. Notably, PV-10 promotes a significant increase in [reactive oxygen species], leading to an elevation in late apoptotic cells. Markers of immunogenic cell death (ICD), including a statistically significant increase in the release of damage-associated molecular pattern molecules HMGB1 and ATP, as well as enhanced surface expression of calreticulin, HSP-70, and HSP-90, were observed. At the molecular level, a remarkable activation of endoplasmic reticulum (ER) stress, pro-apoptotic protein, and autophagy markers were observed. Intratumoral PV-10 injection in vivo has shown significant tumor regression in both mEER and MTE-RAS tumors, and a complete response was noticed in some mice, indicating that PV-10 induces potent ICD in both mEER and MTE-RAS tumors.”
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information provided in this press release may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission, including those described in Item 1A of:
The Company’s Annual Report on Form 10-K for the period ended December 31, 2022, and
Provectus’s Quarterly Report on Form 10-Q for the period ended September 30, 2023.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of Pharmaceutical-Grade Rose Bengal Sodium Abstract for Poster Presentation at 2024 Society for Investigative Dermatology (SID) Annual Meeting
NEWS -- Provectus Biopharmaceuticals, Inc. (PNK:PVCT) Q1 2024 Earnings Call Transcript

Provectus Biopharmaceuticals, Inc. (PNK:PVCT) Q1 2024 Earnings Call Transcript February 22, 2024
Provectus Biopharmaceuticals, Inc. isn't one of the 30 most popular stocks among hedge funds at the end of the third quarter (see the details here).
Operator: Thank you for standing by. This is the conference operator. Welcome to the Provectus Biopharmaceuticals First Quarter 2024 Conference Call. As a reminder, all participants are in listen-only mode and the conference is being recorded. I would now like to turn the conference over to Alyssa Barry, irLabs Investor Relations. Please go ahead.
Alyssa Barry: Thank you, operator. Good afternoon. Welcome to the first quarter 2024 conference call of Provectus Biopharmaceuticals, which is developing immunotherapy medicines for cancer and other diseases. My name is Alyssa Barry, Co-Founder and Principal of investor relations firm irLabs. I am hosting today’s call. Ed Pershing, Chairman of Provectus’ Board of Directors, and Dominic Rodrigues, Board Vice Chairman, will provide company updates and their remarks. First, Nathan Kibler, Provectus’ Outside Legal Counsel from the law firm of Baker Donelson, will read the company’s forward-looking statements.
Nathan Kibler: Thank you, Alyssa. The information provided on this conference call may include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as aim, likely, outlook, seek, anticipate, budget, plan, continue, estimate, expect, forecast, may, will, would, project, projection, predict, potential, targeting, intend, can, could, might, should, believe, and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’ drug product candidates and/or their uses under investigation have not been established. There is no guarantee that these agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels. Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, listeners should not place undue reliance on these forward-looking statements. The forward-looking statements discussed on this conference call are made as of the date hereof or as of the date specifically specified herein, and the company undertakes no obligations to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws.
The forward-looking statements are expressly qualified by this cautionary statement. Risks, uncertainties, and assumptions include those discussed in Provectus’ filings with the U.S. Securities and Exchange Commission, including those described in Item 1A of the company’s Annual Report on Form 10-K for the period ended December 31, 2022, and Provectus’ quarterly report on Form 10-Q for the period ended September 30, 2023.
To continue reading the Q&A session, please click here.
NEWS -- Provectus Biopharmaceuticals Announces First Quarter 2024 Conference Call
KNOXVILLE, TN, Feb. 15, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that it will host a conference call starting at 3 p.m. EST on Thursday, February 22, 2024, to provide Company updates.
The conference call may be accessed by registering in advance or dialing 1-800-319-4610 (in the U.S.) or 1-604-638-5340 (outside the U.S.). Please dial in approximately five minutes prior to the start of the call.
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
The Company’s Annual Report on Form 10-K for the period ended December 31, 2022, and
Provectus’s Quarterly Report on Form 10-Q for the period ended September 30, 2023.
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Engages irlabs for Investor Relations Services
KNOXVILLE, TN, Feb. 14, 2024 (GLOBE NEWSWIRE) -- Provectus Biopharmaceuticals, Inc. (“Provectus” or the “Company”) (OTCQB: PVCT) today announced that it has engaged IR Labs, Inc. (“irlabs”) to develop a comprehensive investor relations and corporate communications program for the Company.
Ed Pershing, Chairman of Provectus’s Board of Directors said, “We are pleased to have irlabs and Alyssa Barry join our team as we seek to increase Provectus’s investor communication and engagement and expand the Company’s visibility and outreach to the investment community.”
“Provectus is a great fit for irlabs and we are excited to help the Company increase its visibility and engagement with retail and institutional investors," said Alyssa Barry, Co-Founder and Principal at irlabs.
About Provectus
Provectus Biopharmaceuticals, Inc. is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes. Provectus’s lead HX molecule is named rose bengal sodium.
Provectus’s medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, full-thickness cutaneous wound healing, and canine cancers; and in vitro discovery programs in infectious diseases, tissue regeneration and repair, and proprietary targets.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
@plegee I agree .75 to $1 easy. Let's see what the mPDAC trial annoucement does to the stock price. I feel very good that the data will hold to to show PV-10 will beat SOC since it has previously on liver mets in other trials and it shouldn't take long once recruitment is done on these type of patients at interim analysis. We may see value increase just with the annoucement.
Merry Christmas!
Isaiah 9:6-7
"For to us a child is born, to us a son is given, and the government will be on his shoulders. And he will be called Wonderful Counselor, Mighty God, Everlasting Father, Prince of Peace. Of the increase of his government and peace there will be no end. He will reign on David's throne and over his kingdom, establishing and upholding it with justice and righteousness from that time on and forever. The zeal of the LORD Almighty will accomplish this."
This stock should be higher than 50 cents at a bare minimum. Recent data read outs, today's news, strong insider support are ample support for higher prices. Don't understand why we are down today on a positive headline.
NEWS -- Provectus Biopharmaceuticals Announces Notice of Allowance of First U.S. Patent of Rose Bengal Sodium for Use as Anticancer and Antiviral Vaccine Adjuvant
KNOXVILLE, TN, Dec. 18, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the United States Patent and Trademark Office (USPTO) has allowed patent application 17/488,430, titled “Halogenated Xanthenes as Vaccine Adjuvants.” The allowed patent application covers the use of Provectus’s pharmaceutical-grade rose bengal sodium (RBS) drug substance as an adjuvant in anticancer, antiviral, and possibly other vaccines to potentially make them work better by enhancing T-cell response.
The allowed application would be Provectus’s first patent award in the field of vaccines from the USPTO. Innovate Calgary, the innovation company of the University of Calgary in Alberta, Canada, is a co-assignee. Aru Narendran, MD, PhD, Professor of Pediatrics, Oncology, Biochemistry and Molecular Biology and Physiology and Pharmacology at the University’s Cumming School of Medicine is a co-inventor. The research underlying the allowed application was led by Dr. Narendran and his lab team (the Narendran Lab).
Preclinical data from ongoing research on the potential use of investigational cancer immunotherapy PV-10 (rose bengal sodium) as a vaccine adjuvant was the subject of a poster presentation by the Narendran Lab at the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting. A copy of the SITC 2023 poster, titled “The iodinated fluorescein derivative PV-10 enhances the antiviral activity of CD8+ T-Cells by inducing STING dimerization: Implications for enhanced vaccine applications,” is available on Provectus’s website at https://www.provectusbio.com/media/docs/2023-SITC-poster.pdf.
The Narendran Lab previously discovered that PV-10 activated stimulator of interferon (IFN) genes (STING), demonstrating its potential as a vaccine adjuvant in PV-10-mediated systemic anti-tumor immune responses. This work, titled “Association of Heat Shock Proteins as Chaperone for STING: A potential link in a key immune activation mechanism revealed by a novel anticancer agent PV-10” was the subject of a poster presentation at the American Association for Cancer Research (AACR) 2020 Virtual Annual Meeting II. A copy of the AACR 2020 poster is available on Provectus’s website at: https://www.provectusbio.com/media/docs/publications/AACR-2020_meeting_posterV3.pdf.
In its SITC 2023 work, the Narendran Lab showed that PV-10 treatment induced STING activation, upregulated cytokines and chemokines, and increased IFN-? secretion by CD8+ T-cells. Dr. Narendran and his colleagues demonstrated PV-10’s ability to function as an effective adjuvant to enhance T-cell responses and concluded that PV-10’s unique modulation of the STING pathway was a potential mechanism of this activity. This work also portends the potential benefit of PV-10 in combination with targeted immunotherapies and antibody-drug conjugates for cancer treatment.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to make pharmaceutical-grade RBS successfully, reproducibly, and consistently at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, wound healing, and animal health; and in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Provides Updated Data on Cancer Immunotherapy PV-10 for Advanced Cutaneous Melanoma
KNOXVILLE, TN, Nov. 15, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today provided updated data from an ongoing Phase 1b/2 clinical trial of investigational cancer immunotherapy PV-10 (rose bengal sodium) in combination with standard of care immune checkpoint blockade (CB) for the treatment of advanced cutaneous melanoma (NCT02557321).
Initial results from CB-naïve patients in the main study cohort illustrated potential clinical benefit across all stages of metastasis. Longer-term follow-up of an expanded patient population has been assessed.
Participants must have had at least 1 injectable lesion and at least 1 measurable target lesion and been a candidate for pembrolizumab. The combination of PV-10 and pembrolizumab was administered every 3 weeks for up to 5 cycles, followed by pembrolizumab alone every 3 weeks for a total duration of up to 24 months. Patients may have received PV-10 as needed (PRN) beyond the initial treatment course per investigator discretion. The primary endpoint of the Phase 1b portion was safety and tolerability. Objective response rate (ORR) and progression-free survival (PFS) were key secondary endpoints (assessed via RECIST 1.1 after 15 weeks, and then every 12 weeks).
The addition of an expansion cohort balanced overall enrollment at 25 patients:
Great news, thank you, was hoping for above average results; PV-10 certainly did not disappoint. Realistically, however, I do not see this doing anything for the price other than today's bump. Some second tier biotech has to be getting interested, however, this has to be compelling to them on so many levels as an affordable investment/partnership. Big pharma does not see the financial opportunity in the PVCT approach; more interested in maintaining their reoccurring price gouging regimens.
We are making progress thank goodness, just do not think this data set will be our start. I believe some collaborative news will eventually be forthcoming, just don't think this release is enough. Added on the last dip to $0.08, will do so again if the opportunity presents itself. The ocular and veterinary products might be the first to make noise.
NEWS -- Provectus Biopharmaceuticals Provides Updated Data on Cancer Immunotherapy PV-10 for Metastatic Uveal Melanoma
KNOXVILLE, TN, Nov. 13, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today provided updated data from an ongoing Phase 1 clinical trial of investigational cancer immunotherapy PV-10 (rose bengal sodium) for the treatment of uveal melanoma (UM) metastatic to the liver (mUM) (NCT00986661).
mUM patients enrolled in this study received 1 or more cycles of PV-10 injection into 1 or more hepatic metastases. Where indicated, standard of care immune checkpoint blockade (CB), as either monotherapy pembrolizumab or the combination of ipilimumab and nivolumab (IN), was also administered.
To date, 25 mUM patients have received monotherapy PV-10 or PV-10 in combination with CB:
NEWS -- Provectus Biopharmaceuticals Announces Presentation of Preclinical PV-10 Vaccine Adjuvant Data at Society for Immunotherapy of Cancer (SITC) 2023 Annual Meeting
KNOXVILLE, TN, Nov. 06, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that preclinical data from ongoing research on the potential use of investigational cancer immunotherapy PV-10 (rose bengal sodium) as an adjuvant in vaccines to help them work better was the subject of a poster presentation at the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting, which was held in San Diego, CA from November 1-5. This research has been led by Aru Narendran, MD, PhD and his lab team from the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
A copy of the SITC poster, titled “The iodinated fluorescein derivative PV-10 enhances the antiviral activity of CD8+ T-Cells by inducing STING dimerization: Implications for enhanced vaccine applications,” is available on Provectus’s website at https://www.provectusbio.com/media/docs/2023-SITC-poster.pdf.
Dr. Narendran and his colleagues previously discovered that PV-10 activated stimulator of interferon (IFN) genes (STING), demonstrating its potential as a vaccine adjuvant in PV-10-mediated systemic anti-tumor immune responses. See Thakur et al., American Association for Cancer Research 2020 Virtual Annual Meeting II.
In its SITC work, the Narendran lab showed that PV-10 treatment induced STING activation, upregulated cytokines and chemokines, and increased IFN-? secretion by CD8+ T-cells. Dr. Narendran and his colleagues demonstrated PV-10’s ability to function as an effective adjuvant to enhance T-cell responses and concluded that PV-10’s unique modulation of the STING pathway was a potential mechanism of this activity.
Dominic Rodrigues, Vice Chair of Provectus’s Board of Directors, said “This novel work introduces the potential of PV-10 to be an effective, multi-purpose, vaccine adjuvant for the first time, and conveys the opportunity to potentially use PV-10 in anti-viral, anti-cancer, and possibly other vaccines for greater protection against disease by improving a person’s immune response to vaccination.”
Mr. Rodrigues added, “We remain grateful to Dr. Narendran and his lab team members for their many-sided research to elucidate PV-10’s various potential mechanisms and applications. Provectus’s preclinical and clinical data to date, which may indicate that the response to PV-10 treatment is tantamount to in situ vaccination, may also suggest that PV-10-adjuvanted vaccines could potentially contribute to more effective and durable immune responses, better responses from patient populations with unique characteristics, higher efficacy using less antigen, and making vaccines more accessible, sustainable, and affordable.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to make pharmaceutical-grade RBS successfully, reproducibly, and consistently at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo development programs in oncology, hematology, wound healing, and animal health; and in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
NEWS -- Provectus Biopharmaceuticals Announces Acceptance of PV-10 Poster Presentation at Society for Immunotherapy of Cancer (SITC) 2023 Annual Meeting

KNOXVILLE, TN, Aug. 07, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that preclinical data from ongoing research on investigational cancer immunotherapy PV-10 (rose bengal sodium) as an immune vaccine adjuvant will be presented on a poster presentation at the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting to be held in San Diego, CA from November 1-5. This PV-10 research has been led by Aru Narendran, MD, PhD and his team of researchers at the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
The accepted abstract is:
NEWS -- Provectus Biopharmaceuticals Establishes Research Collaboration with University of Tennessee College of Veterinary Medicine to Investigate Pharmaceutical-Grade Small Molecule Immunotherapy Rose Bengal Sodium for Canine Soft Tissue Sarcomas

KNOXVILLE, TN, June 28, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company has initiated a new sponsored research program with the University of Tennessee College of Veterinary Medicine (UTCVM) to assess the safety and preliminary efficacy of intralesional injection of a formulation of Provectus’s pharmaceutical-grade RBS for canine soft tissue sarcomas. UTCVM’s lead principal investigator of this work is clinical pathologist, comparative cancer biologist, and Assistant Professor Nora Springer, DVM, PhD, DACVP.
This preclinical and clinical veterinary study is being undertaken as part of the State of Tennessee’s funding to develop animal health drug products in partnership with state universities that have agriculture and veterinary medicine programs and the Company.
Soft tissue sarcomas are common malignant neoplasms in dogs that are locally invasive and have a high prevalence of recurrence after surgical excision. These tumors are often located on the extremities, where complete surgical excision is challenging because minimal soft tissues hinder the ability to gain adequate tumor margins while maintaining the ability to close the surgical wound. Adjuvant chemotherapeutic protocols do not improve either time to recurrence or overall survival with incompletely excised soft tissue sarcomas. Radical measures, such as limb amputation, are often required to ensure complete excision for limb sarcomas and this option is frequently unpalatable to patient owners. Therefore, intralesional injection resulting in tumor ablation may be an ideal treatment modality for soft tissue sarcomas.
Dr. Springer graduated from Marietta College with a Bachelor of Science in Chemistry, LaGuardia Community College with an Associate in Applied Science in Veterinary Technology, Kansas State University with a Doctor of Veterinary Medicine (DVM), and Cornell University with a Doctor of Philosophy (PhD) in Translational Medicine. She completed the American College of Veterinary Pathologists (ACVP) certifying examination to become a Diplomate Member (DACVP), and has co-authored a number of medical journal publications to date.
Dominic Rodrigues, Vice Chairman of Provectus’s Board said, “We are excited to commence this canine cancer study with the University of Tennessee College of Veterinary Medicine and Dr. Nora Springer to accelerate our animal health drug development program. We believe that the Company’s adult cancer clinical and pediatric preclinical data to date can meaningfully contribute to Dr. Springer’s canine cancer work, and vice versa because spontaneous cancers in dogs share many features with human cancers.”
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; proof-of-concept in vivo drug discovery programs in oncology, hematology, wound healing, and animal health; and preclinical in vitro drug discovery programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
Thank you for your write up Wehalls, I remain overall encouraged even though I am a bit deflated that the TGA route seems to be rapidly dwindling. More likely, it appears to be a probable dead end since we would not likely put limited resources into a foreign drug approval agency if a `fast track' avenue is not an option for us.
I always thought the ocular route with Miami would be our first revenue producer despite the statement that Management is "trying to stay focused and putting everything on the back burner that is not related to their current oncology trials". I forgot about the animal/pet possibility, glad to hear that is moving forward, I honestly thought that had great merit as well.
Don't know what to think about the reverse stock split, I do not recall one company off hand that has used it successfully; I realize there are certainly outliers. Sounds like Ed and Dom are not to concerned about financing, that is certainly positive news. And having potential investors helping out with clinical trials is very positive. I like the idea that any financial decision they make has at least some consideration for the shareholders. PHR has been wonderful to us so far, I think they are a stand up management team, no one else would finance a company like they do by buying the convertible shares over market value. Would just like to see some traction in clinical outcomes; just seems like a consistent one foot forward, another step backwards approach.
Appreciate your write-up, I remain pretty upbeat about the company despite knowing it remains a long shot for commercial success (inherent with the industry). One can dream though, and patients may one day may have medical options that can truly extend and improve quality of life thanks to PVCT. Thank you for your efforts.
Might be over 1 to 100 R/S.
I attended the AM and below are my notes from the 2023 Provectus Annual Meeting.
All five directors were present in person this year.
All six proposals approved.
Dominic went over the slides that updated the progress of the company. Slides provided below but you must go past the first few pages to get to the slides. (See slides PVCT sent to investors)
After the presentation, Ed made a few comments, and one interesting remark is that PVCT is going to explore the possibility of having a conference call every 6 months to try and keep the investors more up to date on what’s happening with the company.
The 2022 Reverse Split was discussed and PRH stated that the events they hoped would be in place didn’t happen and they simply decided to not go forward with the R/S.
This is a good place to discuss the TGA event that we all thought would have triggered the R/S. I asked whether an application had been submitted to the TGA and Dominic commented that it was their policy not to comment on these types of actions by management. This is understandable based on previous actions by Dees and their public announcement that they had submitted a package to the FDA which was turned down because of the lack of significant patient data. However, there were comments from other investors in private that they had heard that PVCT had decided to not submit the package because TGA had decided that Intermittent Melanoma was not a rare disease and therefore was not eligible for being fast tracked. Consequently, management decided to not pursue this path of TGA regulatory approval and to go forward with the FDA or maybe with the normal TGA regulatory path (not sure).
There was an interesting discussion about whether or not the delays related to COVID was a blessing or a curse. Management believes that in the long run it was a blessing because it allowed them to open up many different avenues of potential RB use such as dermatology, virology, ophthalmology, and others. They believe without the COVID delays they would have gone down the oncology path and any other venues would not have been explored.
One of my biggest concerns has been funding. Dominic commented that they could go and get the money they needed at a price point that wouldn't necessarily be advantageous to the current stockholders, but that was always an option. They seemed fairly confident that they could find the money they needed to get the trials done without destroying the current stockholders' share position. The bottom line is the money that Dominic and Ed have been putting into the company was not to keep the lights on, but to progress the company's objectives towards regulatory approval by the FDA and maybe the TGA. Also of interest, there are individuals who have the ability to fund specific trials that interest them, and Ed is in discussions with them.
The question was asked regarding Bascom Palmer and negotiations for rights to use their equipment with RB to treat fungal keratitis. Many investors believed that negotiations have broken down. Dominic replied that this was definitely not the case and their relationship with Bascom Palmer was strong and that it was taking longer than anticipated because of the bureaucracy associated with the University of Miami (For Profit University) which is who Bascom Palmer is a part of. He also commented that their equipment is not what is doing the heavy lifting in treating fungal keratitis, but it is PV-10 and there are other equipment options.
A question was asked about operational costs associated with operations and trial costs and Ed commented that the company’s current spend rate is $250K per month.
In regard to the University of Tennessee’s grant for exploring PV-10 with animal sciences, Dr. Lacey stated it has taken a while to find the lead investigator for the study. However, they have now found the individual they need, and she is not only a veterinarian but also a PhD and they have every confidence in her and believe the outcome will be fruitful. In essence, the study is just now getting off the ground, but progress will be made. Unfortunately, the use of PV-10 in animal sciences in Australia will not be of much use in the study other than lessons learned from clinic use and practical applications. The lead investigator will have to start from scratch due to academic rigor although she will have the advantage of the information from the veterinarian’s use of RB in dogs (or other animals) in Australia.
The board of directors stated that they are trying to stay focused and putting everything on the back burner that is not related to their current oncology trials. They are concentrating on pancreatic cancer, metastatic uveal melanoma, stage III melanoma combo, and intransit metastatic melanoma (possibly TGA route, not sure) and will be moving forward with these trials. Also of interest, PVCT is trying to initiate PET scans in their trials because only PET scans are able to determine if the tumor is truly dead. A dead tumor may show up on a regular scan, but the Radiologist can’t tell if the tumor is dead or not and therefore won’t declare a CR without a PET scan. Regarding the date of conclusion of trials, there are a lot of unknowns such as patient recruitment, funding, successful readout of data and application to FDA/TGA, etc. Investor guesses is sometime within the first 6 months of 2024 and that is only a guess.
These are my notes, so if someone else was at the meeting and wants to correct or elaborate on my notes, please feel free to do so and my feelings will not be hurt.
Conclusion: I know we are all very frustrated with the slow pace that this is taking and the low share price and low volume. Management acknowledged our frustration and stated they are also frustrated with the low stock price because they are also stockholders. My impression is that by the next annual meeting, things will be significantly different. I also acknowledge that we have all thought this year after year. I've often said if making money in this stock was easy everybody would be doing it. However, this has been a very difficult investment for all of us and I believe in the long run it could very well be an investment of a lifetime. The Board Members believe PV-10 could be an industry disrupter and could completely change oncology. Not only do we have a great drug, but I believe we have good men on the board of directors. These thoughts are my opinion and based on years of being invested in PVCT, so take it for what it's worth.
NEWS -- Provectus Biopharmaceuticals Announces Stockholder Approval to Undertake Reverse Split of Outstanding Equities and Reduce Number of Authorized Equities by Same Ratio

KNOXVILLE, TN, June 26, 2023 (GLOBE NEWSWIRE) -- Provectus (OTCQB: PVCT) today announced that the Company’s shareholders have approved the proposals of Provectus’s Board of Directors (Board) to seek the authority to undertake a reverse stock split and an authorized share reduction.
At the Company’s Annual Meeting of Stockholders held in Knoxville, Tennessee on June 21, 2023, shareholders also approved the Board’s recommendations of four other proposals: the election of directors, an advisory vote on the approval of the compensation of Provectus’s named executive officers, an advisory vote on the frequency of the aforementioned advisory vote on compensation, and the ratification of the Company’s independent registered public accounting firm.
Ed Pershing, Chairman of Provectus’s Board said, “I want to express my sincere appreciation and the sincere appreciation of our directors, officers, employees, and staff to all of our stockholders for your active participation in the voting process as well as your support of our business direction and management, as evidenced by these favorable voting results. Your continued interest in and support of Provectus is welcomed and greatly appreciated.”
Mr. Pershing added, “The Company’s Board understands its responsibility to evaluate and determine whether and when a reverse stock split of Provectus’s outstanding equities and reduction of authorized shares are in the best interests of the Company and our stockholders.”
A copy of Provectus’s Form 8-K filed on June 22, 2023, which provided details of shareholder voting on the Board’s six proposals and included a brief description of and the vote tabulation for each proposal, may be found here:
https://www.sec.gov/ix?doc=/Archives/edgar/data/315545/000149315223022116/form8-k.htm.
Proposal #1 (election of directors) passed with [an average of] 44% FOR of shares outstanding and 96% FOR of shares voted.
Proposal #2 (advisory vote on compensation) passed with 43% FOR of shares outstanding and 94% FOR of shares voted.
Proposal #3 (one-year frequency of advisory vote on compensation) passed with 43% FOR of shares outstanding and 94% FOR of shares voted.
Proposal #4 (independent accounting firm) passed with 67% FOR of shares outstanding and 99% FOR of shares voted.
Proposal #5 (reverse stock split) passed with 61% FOR of shares outstanding and 90% FOR of shares voted.
Proposal #6 (authorized share reduction) passed with 61% FOR of shares outstanding and 91% FOR of shares voted.
About Provectus
Provectus Biopharmaceuticals, Inc. (Provectus or the Company) is a clinical-stage biotechnology company developing immunotherapy medicines for different diseases that are based on a class of synthetic small molecule immuno-catalysts called halogenated xanthenes (HXs). Provectus’s lead HX molecule is named rose bengal sodium (RBS).
The Company’s proprietary, patented, pharmaceutical-grade RBS is the active pharmaceutical ingredient (API) in the drug product candidates of Provectus’s clinical development programs and preclinical formulations of the Company’s drug discovery programs. Provectus’s pharmaceutical-grade RBS displays different therapeutic effects at different concentrations and can be formulated for delivery by different routes of administration. The International Nonproprietary Names Expert Committee of the World Health Organization selected “rose bengal sodium” for the nonproprietary name of the Company’s API.
RBS may target disease in a bifunctional manner. Direct contact may lead to cell death or repair depending on the disease being treated and the concentration of Provectus’s RBS utilized in the treatment. Multivariate immune signaling, activation, and response may follow that may manifest as stimulatory, inhibitory, or both.
The Company believes that it is the first entity to advance an RBS formulation into clinical trials for the treatment of a disease. Provectus believes that it is the first and only entity to date to successfully, reproducibly, and consistently make pharmaceutical-grade RBS at a purity of nearly 100%.
Provectus’s small molecule HX medical science platform includes clinical development programs in oncology, dermatology, and ophthalmology; in vivo proof-of-concept programs in oncology, hematology, wound healing, and animal health; and in vitro programs in infectious diseases and tissue regeneration and repair.
Information about the Company’s clinical trials can be found at the National Institutes of Health (NIH) registry, https://ClinicalTrials.gov. For additional information about Provectus, please visit the Company’s website at https://www.provectusbio.com.
FORWARD-LOOKING STATEMENTS: The information in this press release may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
#####
Contact:
Provectus Biopharmaceuticals, Inc.
Heather Raines, CPA
Chief Financial Officer
Phone: (866) 594-5999
I'm planning on going to the Annual Meeting on 6/21/23. Anyone else going? If so, several of us investors usually get together for lunch prior to the meeting if you are interested. Let me know and I'll give you the details.
NEWS -- Provectus Biopharmaceuticals Announces Publication of Activity of Pharmaceutical-Grade Rose Bengal Sodium (RBS) Against Colistin-Resistant Gram-Negative Bacteria

Strong today with decent volume at midday. New 20 day high. Wants to push higher.
|
Followers
|
185
|
Posters
|
|
|
Posts (Today)
|
1
|
Posts (Total)
|
9914
|
|
Created
|
08/09/02
|
Type
|
Free
|
| Moderators | |||
.png)

Provectus Biopharmaceuticals is investigating new therapies for the treatment of skin cancer and liver cancer. Provectus investigational oncology drug, PV-10, is an ablative immunotherapy under investigation in solid tumor cancers. The Company has received orphan drug designations from the FDA for its melanoma and hepatocellular carcinoma indications. PH-10, its topical investigational drug for dermatology, is undergoing clinical testing for psoriasis and atopic dermatitis. Provectus has completed Phase 2 trials of PV-10 as a therapy for metastatic melanoma, and of PH-10 as a topical treatment for psoriasis. In addition, Provectus has begun a Phase 3 trial as a therapy for metastatic melanoma. Information about these and the Company's other clinical trials can be found at the NIH registry, www.clinicaltrials.gov. For additional information about Provectus, please visit the Company's website at www.provectusbio.com.
The red synthetic dye Rose Bengal is shown in a bottle at Provectus Pharmaceuticals, Inc.
CURRENT PVCT PIPELINE (as of May 23, 2017)
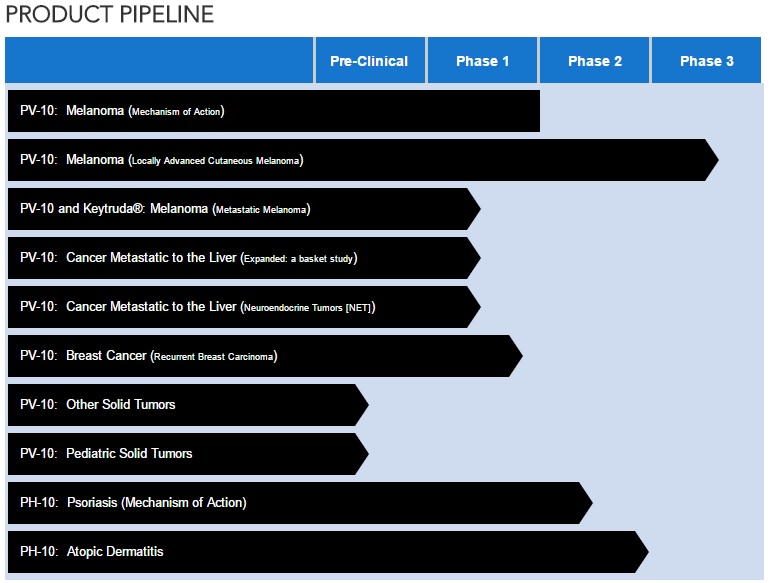
--------------------------------------------------------------
Charts and Technical:
PVCT 6 months chart:
http://stockcharts.com/h-sc/ui?s=PVCT&p=D&yr=0&mn=6&dy=0&id=p03754661229
Technical analysis:
http://www.stockta.com/cgi-bin/analysis.pl?symb=PVCT&num1=7&cobrand=&mode=stock
PVCT News and Analysis:
PVCT News Blog with the latest news and analysis:
http://provectuspharmaceuticalsinc.blogspot.ca/p/news.html
CONNECTING THE DOTS - CURRENT NEWS PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/p/current-news_22.html
CONNECTING THE DOTS - BLOG PAGE
http://provectuspharmaceuticalsinc.blogspot.ca/
PVCT News at OTC
http://www.otcmarkets.com/stock/PVCT/news
CLINICAL TRIALS Updates and Info:
| Short Interest | 167,323 (69.16%) Apr 13, 2017 |
| Significant Failures to Deliver | No |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
