
50B shares increased and completely "SCAM" company.
A COMPLETE SCAM COMPANY WITH 2.4B SHARES AVAILABLE TO DUMP
CEO sells block shares for .005
Let's look at KENNETH WAGGONER CEO OF PHARMACYTE attributes
A CEO of a penny stock
Was associated with 2 scam companies in the past.
Misleads investors with PR wording.
A lawyer, not a businessman and definitely not a Bio Tech person.
Has not been able to generate capital gains for Long term stockholders in PMCB since he joined the company in 2014.
Never has he purchased any shares in PMCB.
PMCB currently has 2.4 billion shares outstanding, without yet submitting an IND.
KW received over 1 million dollars in cash and stock and stock options in 2018, and the company not as yet have an approved product or filed an IND yet:
As Chief Executive Officer, President and General Counsel, Director at PHARMACYTE BIOTECH INC, Kenneth L. Waggoner made $1,004,360 in total compensation. Of this total $375,000 was received as a salary,$312,800 was received in stock options, $316,560 was awarded as stock. This information is according to proxy statements filed for the 2018 fiscal year. A must read about PMCB management
background and previous shadeyness. Well, I've posted this countless times but here you go: PMCB's Oh Wise CEO, Kenneth Waggoner, who had previously worked as EVP for ChinaTel, now VelaTel, which is basically a pump & dump Chinese Reverse Merger stock now worth $.0001 cents. Here's a nice summary of some timely dumping: Quote:"CHTL has certainly treated its stock like an endless money supply. For example, regulatory filings show, CHTL issued more than 30 million shares of stock to consultants and contractors – causing its overhead costs to skyrocket -- during the first nine months of 2009 alone. Since then, those filings show, the company has issued millions of additional shares for similar consulting services. With the stock blowing past $1 near the end of last year, CHTL insiders began to cash in some of their chips. Isidoro Gutierrez, identified in regulatory filings as CHTL’s chief administrative officer and uncle to two of the company’s top executives, sold at the highest prices – fetching up to $1.55 a share – around the time the stock hit its peak. In early February, however, a mysterious Mexican trust holding 4.8% of CHTL’s stock (just short of the 5% stake required for detailed disclosures) reported the most lucrative sales of all. Between Dec. 17 and Jan. 22, the trust sold more than 2 million shares of CHTL – at prices ranging up to $1.91 a share – and pocketed almost $3 million in proceeds in the process. Kenneth Waggoner, CHTL’s outside legal counsel, executed some well-timed sales as well. On the same day thatCHTL announced that Excel had reduced its original funding commitment, for example, Waggoner sold 15,000 shares of stock at just under $1 a share. Two days after CHTL learned that Excel would be unable to make its first big payment – and three days before the company actually disclosed that news – Waggoner followed up with an even bigger sale by cashing in 40,000 shares as the stock headed below 70 cents a share." Quote:San Diego County Superior Court Lawsuit filed March 10, 2010-Case no. 37-2010-00087536-CU-EN-CTL VRT Square LP v. China Tel Group, Mario Alvarez, Kenneth Waggoner, Ken Hobbs, et. al. Complaint alleges causes of action for, among others, Fraudulent Conveyance, Fraud, Conspiracy Unjust Enrichment and Constructive Trust. Allegations state that a legitimate judgment was obtained by VRT Square against Mario Alvarez and his failed company, Greenleaf Home Loans. Complaint further alleges that after obtaining judgment, Mario Alvarez transferred essentially all his assets and all his stock in China Tel Group Inc. to various friends and family members. This case can be viewed at the San Diego Superior Court website From Forbes article: Quote:"The story line is that Gerova and dozens of satellite companies are secretly being manipulated as part of a bid to pump up share prices and dump them on unsuspecting investors—many of whom are effectively required to own Gerova because of its inclusion in the Russell 2000 and 3000 value indexes. Supposedly behind this complex fraud: graduates of Westmoore Capital. Westmoore is a $53 million Ponzi scheme that the SEC shut down in June." Westmoore and Gerova have other connections. The now defunct Westmoore acquired a stake in China Tel Group Inc. (OTCBB: CHTL) on July 29, 2008. That same month Gerova entered into an agreement to acquire China Tel. The Gerova-China Tel deal was unwound three months later. China Tel’s chief executive, George Alvarez, served as a Director of Westmoore Holdings, Inc. until September 25, 2009." Now Waggoner issuing shares to major China Tel Holder Westmoore Capital: Quote: SCHEDULE 13D Under the Securities Exchange Act of 1934 (Amendment No. 0)* China Tel Group, Inc. (Name of Issuer) Series A and Series B Common Stock (Title of Class of Securities) Kenneth L. Waggoner, 12526 High Bluff Drive, Suite 155, San Diego, California 92130, (760) 230-8986 (Name, Address and Telephone Number of Person‘s Authorized to Receive Notices and Communications) Consists of (i) 6,750,000 Series B Shares held by Westmoore Investment L.P., of which Mr. Jennings is general partner; (ii) 2,250,000 Series B Shares held by Westmoore Capital Group Series A, LLC, of which Mr. Jennings is Manager; (iii) 2,036,363 Series B Shares held by Westmoore Management, LLC, of which Mr. Jennings is Chief Executive Officer; (iv) 1,900,000 Series B Shares held by Westmoore Capital Group Series B, LLC, of which Mr. Jennings is Manager; (v) 500,000 Series B Shares held by Westmoore Capital Group Series II, LLC, of which Mr. Jennings is Manager; and (vi) 250,000 Series B Shares held by YYZ Hol Now Krusty Ken associated with another Ponzi player: Quote:April 28, 2009|By Joseph Serna Earlier this month, a Seattle-based firm, Freestone Capital Partners LP and its affiliates, won a judgment against MKA’s Chief Financial Officer Michael Abraham and fund manager Jason Sugarman. MKA did not pay back the money it owed to Freestone, a judge ruled, and Abraham was ordered to pay back $26.3 million plus interest and Sugarman was ordered to pay back $5.97 million plus interest. Several people contacted who said they were clients of MKA have reported investing money, and when they failed to see satisfactory, or any return, on their investments, failed to get their money back when they requested it. The Gateses’ lawsuit alleged the company was operating a make-shift Ponzi scheme to stay afloat, taking on new investors to pay out returns for older ones. Federal authorities declined to comment on any possible investigation into the company. Now Waggoner issuing shares to the very same Jason Sugarman: Quote:Quote:SCHEDULE 13D Under the Securities Exchange Act of 1934 (Amendment No. 0)* China Tel Group, Inc. (Name of Issuer) Series A and Series B Common Stock (Title of Class of Securities) Kenneth L. Waggoner, 12526 High Bluff Drive, Suite 155, San Diego, California 92130, (760) 230-8986 (Name, Address and Telephone Number of Person‘s Authorized to Receive Notices and Communications) I, Jason Sugarman , a shareholder of China Tel Group, Inc., a Nevada corporation (“Corporation”), do hereby irrevocably appoint George Alvarez, to be my proxy agent, with full power of substitution, and to vote all of my shares of Series B common stock in the Corporation with respect to all matters submitted to the shareholders at all meetings of the shareholders, or any adjournments thereof, and in all consents to any actions taken without a meeting. Jens Dalsgaard used to be Nuvilex/efoodsaety's PR rep and was a huge stock fraud. I've posted some of his history before. So the connection is definitely there. Gruden, who bought CiAB, was close to Dalsgaard, and Waggoner was hired by Gruden. Quote: January 23, 2006 09:45 AM Eastern Standard Time SCOTTSDALE, Ariz.--(BUSINESS WIRE)--Jan. 23, 2006--Patricia Ross-Gruden, President and CEO of eFoodSafety, Inc. (OTCBB:EFSF), announced today that eFoodSafety, Inc. has been selected by The KonLin Letter as the #1 Stock Pick for 2006. The KonLin Letter reiterates its "Strong Buy" recommendation of EFSF stock. eFoodSafety, Inc. by Redwood Consultants, LLC Jens Dalsgaard, 415-884-0348 **** (Now for some fun court documentation from around that time:) On April 13, 2009, in northern California District Court, Location Based Technologies (LBAS.OB), filed suit against Jens Dalsgaard, Redwood Investment LLC, and others, under civil RICO laws. Under the Racketeer Influenced Criminal Organization act, individuals who are members of an enterprise that has committed any two of 35 crimes—27 federal crimes and 8 state crimes—within a 10-year period can be charged with racketeering. RICO also permits a private individual harmed by the actions of such an enterprise to file a civil suit; regardless of whether there is a criminal prosecution, and if successful, the individual can collect treble damages. A similar suit, alleging fraud and breach of contract, was brought against Dalsgaard in September, 2008 by Sustainable Power Corporation. Again the dispute centered on transfer of unregistered shares to affiliated parties; the case was settled out of court April 24, 2009 Quote: 16 A. I don't know how they acquired 11,360,000 17 shares of Sustainable Power Corp. I do know, okay, how 18 they defrauded Sustainable Power out of 50 to 60 million 19 shares. 20 Q. Well, before we get to that, tell me what 21 Berkshire Capital Management Company is. 22 A. They're investment bankers supposably. 23 Actually, they are cohorts, partners in crime with 24 Redwood Consultants and Jens Dalsgaard. 25 Q. And Berkshire Capital Management is represented 0094 1 by individuals, I would assume? 2 A. Berkshire Cap -- oh, people that work for them? 3 Q. Yes. 4 A. Oh, that's a great assumption, yes, sir. 5 Q. And who is the head of Berkshire Capital Management Company? 6 A. Well, Joe Fiore would be a pretty good guess, 8 and I would probably bet my life on it. 9 Q. All right. 10 A. So I guess I'm pretty sure it's Joe Fiore. 11 Q. How did you come to meet Mr. Fiore? 12 A. I was introduced to him as my investment 13 banker, okay, the person that would make sure that the 14 company had all the money it needed to do all its 15 business and build all its plants and make sure that, 16 you know, everything we did and all our financial needs 17 would be covered by a Mr. Jens Dalsgaard. 18 Q. Dalsgaard introduced you to Fiore, correct? 19 A. I believe that's what I just said, yes, sir. 20 Q. Okay. Other than Mr. Fiore, can you give me 21 the names of some other people associated with Berkshire 22 Capital Management in a management capacity? 22 Q. And I understand that there's some affiliation 23 with Berkshire -- between Berkshire Capital Management 24 and Eat at Joe's, Ltd.? 25 A. Eat at Joe's, Ltd., is -- gee, Eat at Joe 0096 1 Fiore's would be a better title. 2 Q. It's another company that Mr. Fiore owns? 3 A. That is correct. 4 Q. And Diamond Ranch, Ltd., is another one of 5 those entities? 6 A. Can I talk to my attorney for a second? Quote: On January 7, 2016, PharmaCyte Biotech, Inc., a Nevada corporation ("Company"), entered into a Stock and Warrant Purchase Agreement with each of Berkshire Capital Management Co., Inc. and SPYR (Joe Fiore), Inc. (collectively, "Investors") and closed a private placement to the Investors of restricted shares of the Company's common stock ("Common Stock") and warrants ("Warrants") to purchase Common Stock ("Private Placement"). Share Structure
Market Cap 
33,854,751
01/15/2021
Authorized Shares
2,490,000,000
01/04/2021
Outstanding Shares
2,334,810,405
01/04/2021
Unrestricted
Not Available
Held at DTC
2,211,876,348
01/04/2021
Float
2,228,380,956
08/25/2020
https://www.otcmarkets.com/stock/PMCB/security OTHER MOD CAN ADD ANYTHING BELOW MY LINE BUT DO NOT EDIT
OR DELETE MY INTRO ------------------------------------------------------------------------------------------Other MOD ……………………………………………………………………………………………………………>..................
the following material is added to the intro by moderator 1234jklm.
Pharmacyte Biotech has developed Targeted 'surgical'
chemotherapy without side effects and has submitted its
IND to the FDA for a phase 2b clinical trial for late stage
Inoperable Pancreatic Cancer; an unmet medical need.
- supported by some of the world's most eminent oncologists and cellular biologists.
- Orphan Drug designation from drug regulatory authorities in the U.S. & EU.
- Principal Trial Investigator is Dr Manuel Hidalgo; the Chief of the Division of
Haematology and Medical Oncology ar Weill Cornell Medicine and New York
Presbyterian /Weill Cornell Medical Centre
PharmaCyte Biotech Announces IND Submitted to U.S. FDA for Clinical Trial
in Locally Advanced, Inoperable Pancreatic Cancer
September 02, 2020
LAGUNA HILLS, Calif.--(BUSINESS WIRE)-- PharmaCyte Biotech, Inc. (OTCQB: PMCB), a clinical-stage biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that it has submitted an Investigational New Drug application (IND) to the U.S. Food and Drug Administration (FDA) for a planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, commented, “Submitting this IND is the most important milestone the Company has met thus far in the clinical development of our leading product candidate. That’s our therapy to combat what is truly a global unmet medical need for those patients with LAPC whose tumors no longer respond to the first line treatments of either Abraxane® plus gemcitabine or FOLFIRINOX.
“Now that the IND has been submitted, we must wait a minimum of 30 calendar days before initiating our clinical trial. During this time, the FDA has an opportunity to review the IND to ensure that it’s complete and that the planned clinical trial research patients will not be subject to unreasonable risk. It also gives the FDA time to ask for more information and clarification about the information submitted.
“Completing and submitting our IND is the culmination of many years of hard work and dedication to ensure that we’ve dotted every ‘I’ and crossed every ‘T’ by our partner, Austrianova, all of our committed consultants and our team at PharmaCyte, including our Chief Operating Officer, Dr. Gerald W. Crabtree, our Consulting Chief Medical Officer, Dr. José Iglesias, and the Chairman of our Medical and Scientific Advisory Board, Dr. Matthias Löhr.”
PharmaCyte’s Consulting Chief Medical Officer, Dr. José Iglesias, stated, “I am pleased that PharmaCyte has submitted its IND to the FDA. The entire PharmaCyte team can now begin work to prepare for the clinical trial. Combining a unique biologic that consists of encapsulated genetically altered human cells with a well-known cancer drug to treat LAPC, PharmaCyte has the possibility of changing the way LAPC is treated in the future. This novel treatment modality may also hold the potential for extending the life span of patients with LAPC.”
The proposed multicenter, randomized, open-label Phase 2b clinical trial is intended to evaluate the efficacy and safety of CypCaps™ (genetically engineered human cells encapsulated using the Cell-in-a-Box® technology) in combination with low doses of the chemotherapy prodrug, ifosfamide, as compared to chemoradiation therapy with capecitabine plus external beam radiation therapy (“EBRT”) or stereotactic body radiation therapy (“SBRT”) alone. The study population will consist of approximately 100 patients. Patients will be randomized in a 1:1 ratio to either treatment with the study therapy or a comparator. The randomization will be stratified by previous treatment (Abraxane® plus gemcitabine or FOLFIRINOX) and the control arm choice (capecitabine/EBRT or SBRT alone).
The primary objective will be determined by progression free survival (“PFS”). The secondary objectives for this study are to determine if CypCaps™ plus low-dose ifosfamide will: (i) increase overall survival (“OS”); (ii) increase objective response rate; (iii) increase the rate of conversion of the pancreatic tumor from inoperable to operable; (iv) decrease the pancreatic cancer tumor marker CA 19-9; and (v) improve a patient’s quality of life. In addition, this clinical trial will assess the safety and tolerability of CypCaps™ plus low dose ifosfamide.
Principal Investigator: Dr Manuel Hidalgo
Chief of the Division of Hematology and Medical Oncology at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center
The E. Hugh Luckey Distinguished Professor of Medicine, Dr. Hidalgo comes to Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center from Beth Israel Deaconess Medical Center in Boston, where he served as chief of the Division of Hematology as well as clinical director of the Rosenberg Clinical Cancer Center. He is also the Theodore W. and Evelyn G. Berenson Professor of Medicine at Harvard Medical School and deputy associate director for clinical sciences at the Dana-Farber/Harvard Cancer Center.
A respected oncologist and premier investigator, Dr. Hidalgo’s work has centered on translational and clinical research in anticancer drug development with a particular emphasis in gastrointestinal cancers. He has developed, tested and helmed the early clinical development of more than 50 new anticancer agents for pancreatic and other solid tumor cancers. Three of those agents, including nab-paclitaxel, are now approved by the U.S. Food and Drug Administration for treatment of GI cancers, including pancreatic cancer. His team also pioneered the development of a personalized cancer model using mice, called mouse avatars, to pre-test cancer treatments.
"Under Dr. Hidalgo’s direction and working with the Joint Clinical
Trials Office at Weill Cornell Medicine and NewYork-Presbyterian/
Weill Cornell Medical Center, the division will enhance its already
robust clinical trials portfolio, which takes the innovative approaches
to cancer therapy from the lab to the patient.
Our priority is ensuring that our clinical trials portfolio is modern,
comprehensive and diverse,” he said, “so patients with advanced
cancers have the opportunity to receive the latest therapies.”
Consulting Chief Medical Officer: José L. Iglesias M.D.
Dr. Iglesias brings a wealth of experience in developing and testing a variety of cancer chemotherapeutic agents to PharmaCyte, including key positions with many prominent biotechnology firms including Eli Lilly, Amgen, Abraxis and Celgene. Dr. Iglesias’s body of work is ideally suited to guide PharmaCyte through its planned Phase 2b clinical trial in LAPC. As the global Vice-President of Clinical Development at Celgene, Dr. Iglesias was the lead physician on the team that obtained FDA approval for Abraxane® (the nab-paclitaxel/gemcitabine combination), which is a first-line therapy in pancreatic cancer.
Chairman and a founder of Austrianova: Prof. Walter H. Günzburg, Ph.D.
The Professor of Virology at the University of Veterinary Medicine, Vienna, Austria and formerly an Adjunct Professor at the National University of Singapore and director of the Christian Doppler Laboratory for Gene Therapeutic Vector Development in Vienna.
Prior to founding Austrianova, he was the Chairman of the board of Austrianova Biotechnology GmbH and Austrianova Biomanufacturing AG. He has also been an advisor to Paktis (Germany) and Tocagen (USA), as well as a founder, board member and former Chairman of Virusure GmbH, a virus testing company located in Vienna, Austria.
He has also served as a member of German Federal Medical Association Ethics Committee for Gene Therapy (Kommission Somatische Gentherapie der Bundesärztekammer) since 1997 as well as a member of the European Academy for the Study of Consequences of Scientific and Technological Advances (since 1998).
Prof. Günzburg has more than 30 years experience in virology, and twenty years experience in cell therapy and bioencapsulation. He has published numerous peer reviewed scientific publications.
Product Manager of Global research for the Biosciences Division of ThermoFisher Scientific: David Judd
David has worked for ThermoFisher for 24 years in cell culture products and applications. David has a broad array of experience in development of media for primary cells and cell lines. He has developed manufacturing processes, cell assays, biochemical analysis, cell culture processes and downstream recovery strategies. He has stepped out of the lab in order to find new ways to provide new workflow solutions for serum products for cell culture applications.
Targeted Chemotherapy
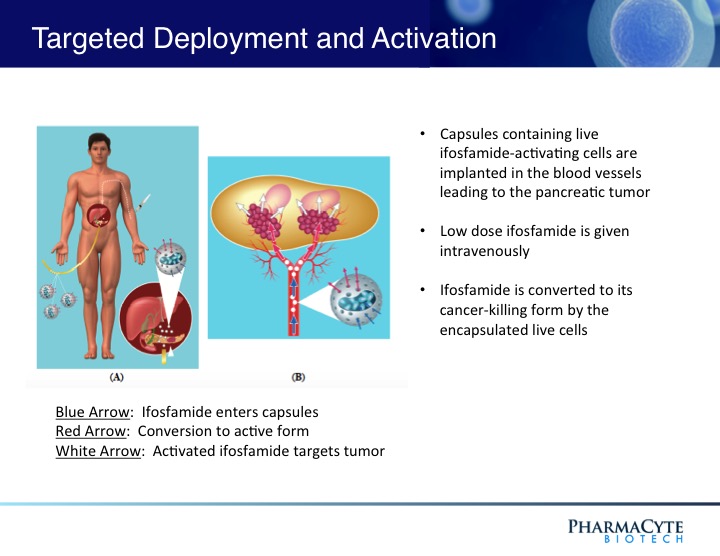
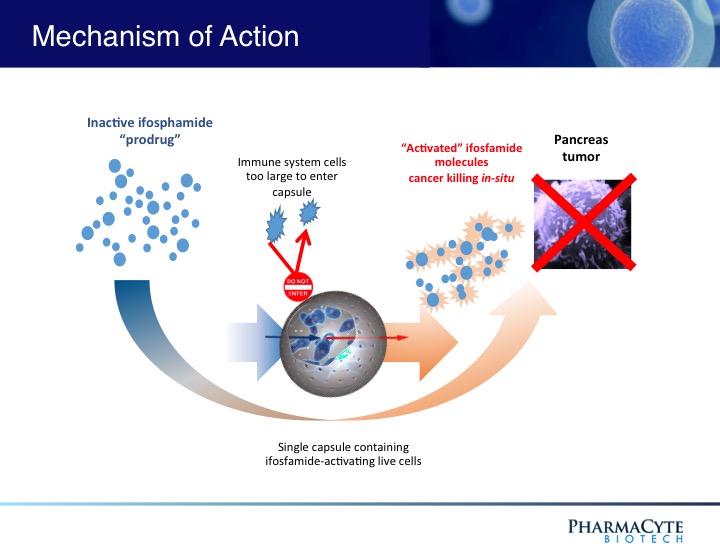
We are employing the Cell-in-a-Box® technology as a platform to develop therapies for solid cancerous tumors using targeted chemotherapy. Our live-cell encapsulation technology-based therapies consist of encapsulating different types of genetically modified living cells depending on the disease being treated. For our leading product candidate, a therapy for pancreatic cancer, about 10,000 genetically modified live cells that produce an enzyme which converts the chemotherapy prodrug ifosfamide into its cancer-killing form are encapsulated in porous, spherical, pinhead-sized capsules, composed largely of cellulose. About 300 of these capsules are placed in the blood supply as close to the tumor in the pancreas as possible. Then about one-third the normal dose of the chemotherapy prodrug ifosfamide is given to the patient intravenously. The prodrug is normally activated in the patient’s liver. By activating the prodrug near the tumor using the Cell-in-a-Box® capsules, our cellular therapy acts as a type of “artificial liver.” Using this “targeted chemotherapy,” we are seeking to create an environment that enables optimal concentrations of the “cancer-killing” form of ifosfamide at the site of the tumor. Because the cancer-killing form of ifosfamide has a short biological half-life, we believe that this approach results in little to no collateral damage to other organs in the body. We also believe this treatment significantly reduces tumor size with no treatment-related side effects.
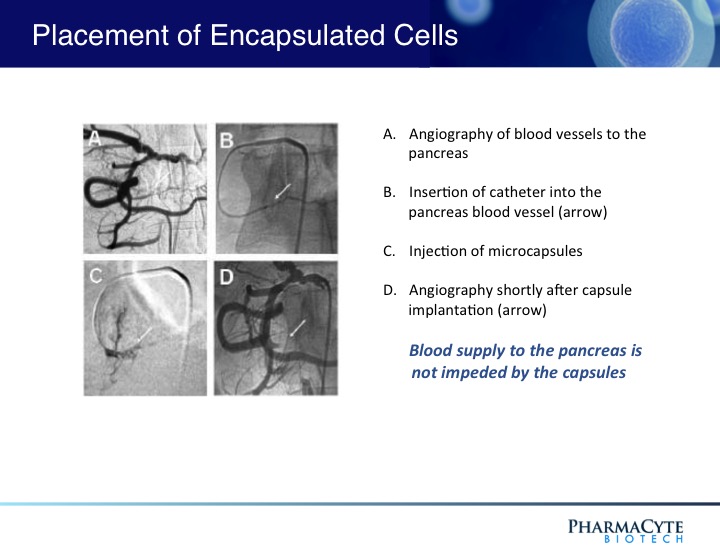
As shown above, the presence of the capsules in the blood vessel leading to the tumor do not prevent the blood flow to the tumor.

Since the cancer-killing metabolite of ifosfamide has a short half-life, we believe will result in little to no collateral damage to other organs in the body. In an earlier Phase 1/2 clinical trial which used our pancreatic cancer treatment in very sick patients with advanced pancreatic cancer, this targeted chemotherapy not only reduced the tumor size but also resulted in no obvious adverse side effects attributable to the therapy.
Orphan Drug designation from drug regulatory authorities in the U.S. and the E.U.carries with it 7 years of marketing exclusivity in the U.S. and 10 years in the E.U. after the product has been approved for marketing by these regulatory authorities.
Pancreatic Cancer
Pancreatic cancer is a very aggressive cancer with a poor prognosis. It is the third leading cause of cancer-related deaths in the U.S. and the seventh leading cause of cancer-related deaths globally. The five-year survival rate is 8%, reportedly the lowest survival rate of any cancer. It is expected that in 2017 there will be more than 53,000 new cases diagnosed in the U.S. In Europe, the estimate is approximately 80,000. Unfortunately, about 72% of patients will die within the first year of diagnosis. More than 90% will die within two years of diagnosis. Without treatment after diagnosis, patients have a 3-6 month median life expectancy.
Patients with pancreatic cancer are not normally diagnosed until the cancer is advanced and inoperable. There is no cure for pancreatic cancer unless the tumor is surgically removed in its earliest stages of growth. Since the first drug (gemcitabine) was approved in the U.S. for pancreatic cancer in 1996, approximately 40 registrational clinical trials have been conducted. In spite of this, little improvement in median survival time and percentage of one-year survivors has occurred. Most success was achieved when gemcitabine was given in combination with another chemotherapy drug.
The current standard of care for advanced pancreatic cancer is the combination of Abraxane® (a nanoparticle albumin formulation of the drug paclitaxel (Taxol®) plus gemcitabine or the 4-drug combination known as FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin). Abraxane® plus gemcitabine was approved by the U.S. Food and Drug Administration (“FDA”) in September 2013. This combination increased the median survival time by 1.8 months, as compared to gemcitabine alone. It increased the one-year survival time from 22% with gemcitabine alone to 38% with Abraxane® plus gemcitabine. FOLFIRINOX has never been approved by the FDA or any other regulatory agency such as the European Medicines Agency (“EMA”), but was introduced to clinical practice in 2010 due to the results of a Phase 2/3 trial comparing FOLFIRINOX with the single-agent gemcitabine as first-line treatment for patients with metastatic pancreatic cancer. Median overall survival was superior with FOLFIRINOX over gemcitabine. Since the conclusion of that trial, oncologists in Europe began using it as first line therapy. That trend has increased in the U.S. despite the FDA approved combination of Abraxane® plus gencitabine. However, there are severe side effects associated with each chemotherapy combination.
PharmaCyte’s Pancreatic Cancer Therapy
A critical unmet medical need exists for patients with locally advanced, unresectable, non-metastatic pancreatic cancer (“LAPC”) who no longer respond to first-line therapy with either Abraxane® plus gemcitabine or the 4-drug combination known as FOLFIRINOX – the current first-line standards of care for advanced pancreatic cancer. These patients have no effective treatment alternative once their tumors no longer respond to these therapies. Two of the most commonly used treatments are 5-fluorouiracil (“5-FU”) or capecitabine (a prodrug of 5-FU) plus radiation. Both treatments are only marginally effective in treating the tumor and result in serious side effects. Other treatments are being tried in an attempt to address this problem, but their success is far from certain. We are developing a therapy that we believe can serve as a “consolidation therapy” with the current standards of care and address the critical unmet medical need discussed above.
Subject to FDA approval, we plan to commence a clinical trial involving patients with LAPC whose tumors have ceased to respond to either Abraxane® plus gemcitabine or FOLFIRINOX. We had a Pre-Investigational New Drug Application (“Pre-IND”) meeting with the Center for Biologics Evaluation and Research of the FDA (“CBER”) in January 2017. At the Pre-IND meeting, the FDA communicated its agreement with certain aspects of our clinical development plan, charged us with completing numerous tasks and provided us with the guidance on the tasks we need to complete for a successful Investigational New Drug Application (“IND”). The proposed clinical trial is designed to show that our Cell-in-a-Box® plus low-dose ifosfamide therapy can serve as an effective and safe consolidation chemotherapy for patients whose tumors no longer respond after four to six months of therapy with Abraxane® plus gemcitabine or FOLFIRINOX. The trial will initially take place in the United States (“U.S.”) with possible study sites in Europe later in the trial.
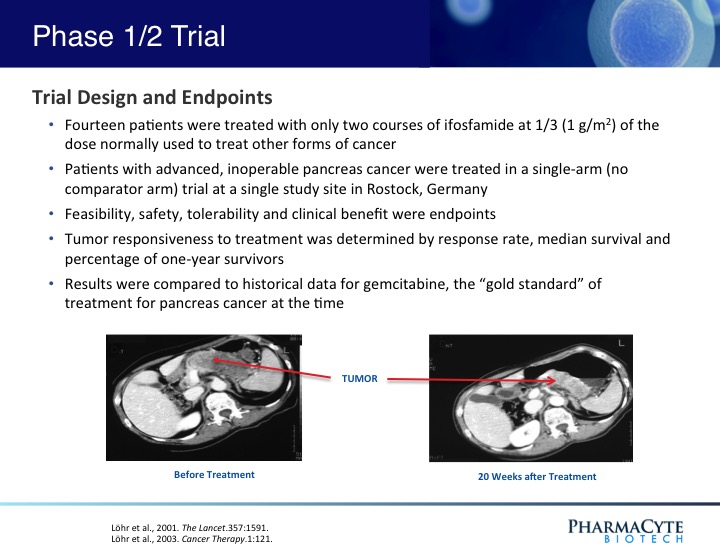
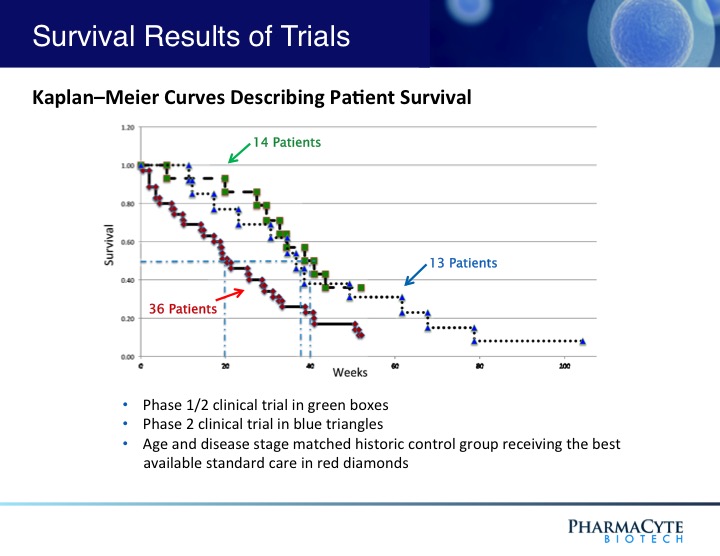
In a previous Phase 1/2 open-label, single site 14-patient clinical trial using our therapy and in which only two courses of ifosfamide were given, our treatment was shown to be safe and effective.(See Figure 3). In fact, when the results of that trial were compared to historical data for gemcitabine (the “gold standard” for pancreatic cancer at the time that this trial was conducted) the percentage of one-year survivors was double that previously reported for gemcitabine, the median survival of patients was substantially increased as compared to gemcitabine, and in contrast to gemcitabine, no treatment-related side effects were seen with our therapy. Interestingly, in 3 of the 14 patients, their advanced-stage tumors were reduced in size to the point where they would have been operable.
PharmaCyte’s Trial Design
The trial will be a prospective, multicenter, randomized, open-label Phase 2b study to evaluate the efficacy and safety of CypCapsTM (genetically engineered human cells encapsulated using the Cell-in-a-Box® technology) in combination with low-dose ifosfamide as compared to chemoradiation therapy with capecitabine plus external beam radiation therapy (“EBRT”) or stereotactic body radiation therapy (“SBRT”) alone. The study population will consist of approximately 100 patients. Patients will be randomized in a 1:1 ratio to either treatment with the study therapy or a comparator. The randomization will be stratified by previous treatment (Abraxane® plus gemcitabine or FOLFIRINOX) and the control arm choice (capecitabine/EBRT or SBRT).
The primary objective will be determined by progression free survival (“PFS”). The secondary objectives for this study are to determine if CypCaps plus low-dose ifosfmide will: (i) increase overall survival (“OS”); (ii) increase objective response rate; (iii) increase the rate of conversion from unresectable to resectable pancreatic cancer; (iv) decrease the pancreatic cancer tumor marker CA 19-9; and (v) improve a patient’s quality of life. In addition, this study will assess the safety, tolerability and immunological response of CypCaps plus low-dose ifosfamide.
Breast Cancer
About 1 in 8 U.S. women will develop invasive breast cancer over the course of her lifetime. In 2018, an estimated 266,120 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S., along with 63,960 new cases of non-invasive (in situ) breast cancer. About 2,550 new cases of invasive breast cancer are expected be diagnosed in men. Approximately 40,920 women in the U.S. are expected to die in 2018 from breast cancer, though death rates have been decreasing since 1989. These decreases are thought to be the result of treatment advances, earlier detection through screening and increased awareness. However, for women in the U.S., breast cancer death rates are higher than those for any other cancer besides lung cancer. Worldwide, in 2018 there were over 2 million new cases of breast cancer diagnosed. Also, breast cancer is the most commonly occurring cancer in women throughout the world and the second most common cancer overall.
PharmaCyte Breast Cancer Treatment
According to the American Cancer Society, ten different chemotherapeutic drug combinations have been widely used for years to treat various forms of breast cancer. Nine of these ten combination chemotherapies use the anticancer drug cyclophosphamide (Cytoxan®) as one of the components of the combination.
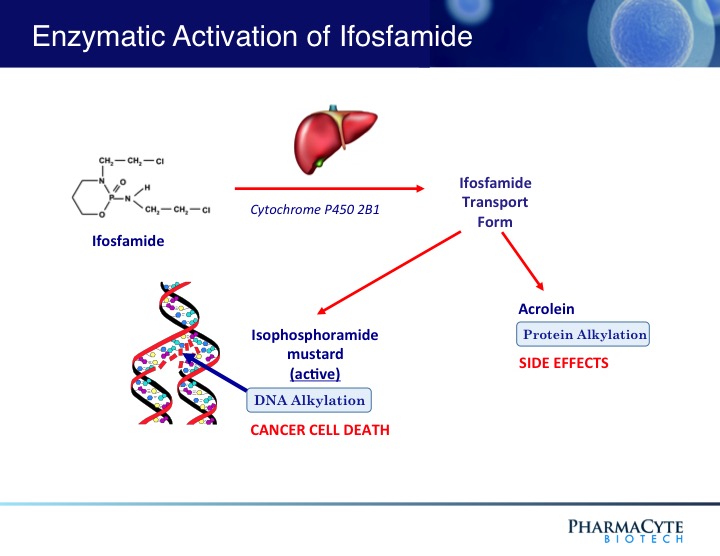
Cyclophosphamide is a “sister” drug to ifosfamide – the anticancer agent used in the clinical trials in patients with advanced, inoperable pancreatic cancer using PharmaCyte Biotech’s treatment for pancreatic cancer. Both drugs are classified as “prodrugs” and must be activated (converted to their cancer-killing form) for them to be effective. The cytochrome P450 enzyme system in the liver activates both cyclophosphamide and ifosfamide.
For PharmaCyte’s pancreatic cancer treatment, the cells that are encapsulated using the Cell-in-a-Box® technology
contain an isoform (known as CYP2B1) of the cytochrome P450 enzyme system and are efficient at activating ifosfamide. Because of the similarities between ifosfamide and cyclophosphamide, the same encapsulated cells as those used in the pancreatic cancer clinical trials should efficiently and effectively activate cyclophosphamide into its cancer killing form in developing a novel treatment for breast cancer.
Veterinary Phase 1/2 Trial in Dogs with Breast Cancer
To test this hypothesis, Cell-in-a-Box® capsules containing cells with high CYP2B1 activity were used in combination with cyclophosphamide in a veterinary Phase 1/2 veterinary clinical trial in dogs with spontaneously-occurring mammary tumors. This is a good animal model system for testing the effectiveness of cyclophosphamide in treating breast cancer in humans because the development and pathological features of mammary tumors in dogs are very similar to those seen in breast cancer in women. Spontaneous mammary tumors account for about 70% of all cancers in dogs. Usually such tumors are removed surgically, but it has been estimated that more than 55% of dogs develop new cancers after such surgery. Cyclophosphamide was chosen for this veterinary Phase 1/2 trial rather than ifosfamide because it is often used to treat breast cancer in women as well as mammary tumors in dogs. In addition, as noted above, it is a component of most combination chemotherapy regimens used against breast cancer in humans.
A total of 16 dogs were enrolled in the preclinical trial. The dogs were divided into two groups. One group of 6 dogs was treated intravenously with cyclophosphamide alone at a dose of 7 mg/kg of body weight, in four treatments on days 2, 9, 22, and 29. In the 10 other dogs, 20 Cell-in-a-Box® capsules containing the cyclophosphamide-activating cells were implanted at 5 sites around their tumors on day 0 (two days before administration of the first dose of cyclophosphamide) and then cyclophosphamide was administered in the same regimen as the other 6 dogs. Three of the 10 dogs treated with the encapsulated cells/cyclophosphamide combination had two tumors as did one dog treated with cyclophosphamide alone.
As in the pancreatic cancer clinical trials in humans, the capsules and the cells within them were well tolerated – no “safety” issues were seen in the dogs that could be related to the presence of the capsules or to their implantation over the entire 56-day observation period. Although mild side effects from the use of cyclophosphamide were observed in both groups of dogs, these were no more severe than those seen when this drug is used alone to treat mammary cancer in canines.
The final evaluation of the effect of the treatments on tumor size were graded as follows: Complete remission (CR), Partial remission (PR) – regression of the tumor by at least 50% of the initial tumor mass, Stable disease (SD) – regression of the initial tumor mass by less than 50% or its enlargement by less than 25%, Progression of disease (PD) – increase in initial tumor mass of at least 25% or the occurrence of new tumors. Six of the 11 tumors treated with the encapsulated cells/cyclophosphamide combination showed a PR while the other 5 showed SD. Of the 7 tumors in the 6 dogs that received cyclophosphamide alone, 6 tumors showed SD and the other exhibited PD. The median tumor size reduction of the tumors in dogs treated with the combination was 53%. This is in contrast with only a 21% reduction of the size of the tumors in the dogs treated with cyclophosphamide alone.
For one of the dogs with two tumors in the combination-treated group, both tumors showed a similar reduction in size (65% and 62%). In a second dog with two tumors in this group, one tumor that received the combination treatment was reduced in size by 70% while the other tumor, which was exposed to cyclophosphamide alone, was reduced in size by only 14%. The tumors in dogs that were administered cyclophosphamide alone were reduced in size by only about 19%.
The results of this study indicate that the combination of the Cell-in-a-Box® cellulose-based live-cell encapsulation and cyclophosphamide may prove to be of significant benefit not only for the treatment of mammary cancers in the veterinary setting, but also may ultimately find a role in the treatment of breast cancer in humans by optimizing the cancer-killing activity of cyclophosphamide when that drug is used for breast cancer
Live Cell Encapsulation Technology
The Cell-in-a-Box® live cell encapsulation technology employed by PharmaCyte is a “platform” upon which treatments for different diseases may be built. This technology encloses live cells in protective “capsules” about the size of the head of a pin. The capsules are designed to allow blood to enter and nourish the living cells inside them. The live cells thrive while the capsules are in the body. But the capsules are also designed to protect the live cells from attack by the body’s immune system. The live cells are too large to escape from the capsules, and the body’s immune system cells are too large to enter the capsules and destroy them. PharmaCyte is using this technology to develop unique therapies for various forms of cancer and for Type 1 diabetes and insulin dependent Type 2 diabetes.
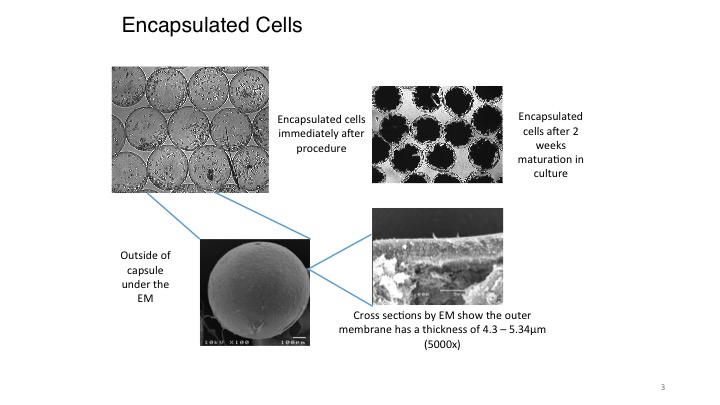
The encapsulation of living cells using the Cell-in-a-Box® technology is a multi-step process. To be encapsulated, the live cells are first suspended in a medium that contains a proprietary polymer and sodium cellulose sulfate. This suspension is passed through a droplet-forming machine, and the resulting droplets fall into a solution containing another polymer. As the two polymers interact, immediately a membrane forms around each droplet. This develops into a shell around the droplet, resulting in a spherical capsule 0.7-0.8 mm in diameter. For pancreatic cancer, each capsule contains about 10,000 living cells. This number of live cells can differ depending upon the size of the cells encapsulated and for what purpose they are to be used.
Encapsulation Material
The Cell-in-a-Box® capsules have been shown to be safe, effective and durable in humans for approximately two years during two early clinical trials. The capsules are made of bio-inert material (cellulose/cotton). They have pores for nutrient and waste transfer. The pores are too small for the immune system cells to enter or the encapsulated live cells to leave. Also, the live encapsulated cells can be frozen and later thawed with viability of approximately 90% or more upon thawing. Hence, the frozen encapsulated cells have a long-term (5+ years) shelf life which results in manageable logistics.
The capsules produced using the Cell-in-a-Box® encapsulation differ from those produced by others. Our competitors use substances such as alginate (a seaweed derivative) or chitosan, whereas our capsules are made principally of cellulose – a bio-inert material in the human body. The capsules durable, resilient and long-lasting. They can also withstand various external forces unlike those made using other encapsulation methods. The properties of our capsules allows them to be implanted in the human body by using needles or catheters without damage to the human body. Our capsules do not degrade, even after being present in the body for over two years. They do not cause any damage to or inflammation of tissues where they are located in the body.


Latest news...
The FDA’s Center for Biologics Evaluation and Research (CBER) successfully processed PharmaCyte’s DMF
August 5th 2020
PharmaCyte Biotech, Inc. (OTCQB: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that the Drug Master File (DMF) that its partner, Austrianova, filed with the U.S. Food and Drug Administration (FDA) has been accepted without any questions or suggested changes.
The FDA’s Center for Biologics Evaluation and Research (CBER) successfully processed PharmaCyte’s DMF, which provides all confidential and detailed information covering the production of the CypCaps™ final product that was produced by Austrianova and will be used in PharmaCyte’s planned clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, said, "CBER’s acceptance and processing of our Drug Master File without any questions or comments is a credit to the painstaking work and dedication by Austrianova and our consultants to ensuring that the entire process is properly conducted and completed before any further documents are submitted to the FDA.
"We now look forward to presenting the FDA with our Investigational New Drug Application very soon for our planned clinical trial in locally advanced, inoperable pancreatic cancer."
A DMF is submitted to the FDA to provide detailed information about facilities, processes and materials used in the manufacturing, processing and packaging of human drugs. It is a prerequisite to securing approval and commercialization and ensures confidentiality of proprietary information related to the Active Pharmaceutical Ingredient (API). The information contained in a DMF is used to support, among other things, an IND.
The DMF requirements are complex and specific, encompassing every detail involved with the manufacture of the API – from raw materials to analytical methods, process development and optimization. The scrutiny goes all the way back to the starting materials used in the API.
José L. Iglesias M.D. appointed as Consulting Chief Medical Officer (CMO) for its planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
August 10
PharmaCyte Biotech, Inc. (OTCQB: PMCB), a biotechnology company focused on developing targeted cellular therapies for cancer and diabetes using its signature
live-cell encapsulation technology, Cell-in-a-Box®, today announced that it has appointed José L. Iglesias M.D. as Consulting Chief Medical Officer (CMO) for its planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
Dr. Iglesias brings a wealth of experience in developing and testing a variety of cancer chemotherapeutic agents to PharmaCyte, including key positions with many prominent biotechnology firms including Eli Lilly, Amgen, Abraxis and Celgene. Dr. Iglesias’s body of work is ideally suited to guide PharmaCyte through its planned Phase 2b clinical trial in LAPC. As the global Vice-President of Clinical Development at Celgene, Dr. Iglesias was the lead physician on the team that obtained FDA approval for Abraxane® (the nab-paclitaxel/gemcitabine combination), which is a first-line therapy in pancreatic cancer.
Dr. Iglesias is also familiar and experienced with the treatment of various abdominal cancers and the use of gemcitabine in patients. He was instrumental in the design of the Phase 3 clinical trial for the development of nab-paclitaxel for use against metastatic pancreatic cancer while at Celgene.
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, stated, “We are extremely fortunate to have Dr. Iglesias join PharmaCyte for our planned Phase 2b clinical trial in LAPC. We interviewed a number of impressive candidates, but his experience in dealing with various cancer chemotherapeutic agents and, more importantly, his direct knowledge in treating pancreatic cancer make him the obvious choice for the position of Consulting CMO for our clinical trial.
I would be remiss in not saying that Dr. Manuel Hidalgo, who will be the Principal Investigator for our planned clinical trial in LAPC, personally recommended Dr. Iglesias to us for consideration. Dr. Hidalgo considers Dr. Iglesias to be one of the most brilliant physicians in the field.”
---------
PharmaCyte Biotech (PMCB) said Wednesday it will broaden its product pipeline to include programs in diabetes and malignant ascites after raising $90 million.
"With the closing of two separate public offerings totaling approximately $90 million now complete, PharmaCyte can, for the first time in its history, operate comfortably in its development of treatments for hard-to-treat diseases without being constrained by financial resources," Chief Executive Kenneth Waggoner said.
Pharmacyte Biotech has developed Targeted 'surgical'
chemotherapy without side effects and has submitted its
IND to the FDA for a phase 2b clinical trial for late stage
Inoperable Pancreatic Cancer; an unmet medical need.
- supported by some of the world's most eminent oncologists and cellular biologists.
- Orphan Drug designation from drug regulatory authorities in the U.S. & EU.
- Principal Trial Investigator is Dr Manuel Hidalgo; the Chief of the Division of
Haematology and Medical Oncology ar Weill Cornell Medicine and New York
Presbyterian /Weill Cornell Medical Centre

PharmaCyte Biotech Announces IND Submitted to U.S. FDA for Clinical Trial
in Locally Advanced, Inoperable Pancreatic Cancer
September 02, 2020
LAGUNA HILLS, Calif.--(BUSINESS WIRE)-- PharmaCyte Biotech, Inc. (OTCQB: PMCB), a clinical-stage biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that it has submitted an Investigational New Drug application (IND) to the U.S. Food and Drug Administration (FDA) for a planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, commented, “Submitting this IND is the most important milestone the Company has met thus far in the clinical development of our leading product candidate. That’s our therapy to combat what is truly a global unmet medical need for those patients with LAPC whose tumors no longer respond to the first line treatments of either Abraxane® plus gemcitabine or FOLFIRINOX.
“Now that the IND has been submitted, we must wait a minimum of 30 calendar days before initiating our clinical trial. During this time, the FDA has an opportunity to review the IND to ensure that it’s complete and that the planned clinical trial research patients will not be subject to unreasonable risk. It also gives the FDA time to ask for more information and clarification about the information submitted.
“Completing and submitting our IND is the culmination of many years of hard work and dedication to ensure that we’ve dotted every ‘I’ and crossed every ‘T’ by our partner, Austrianova, all of our committed consultants and our team at PharmaCyte, including our Chief Operating Officer, Dr. Gerald W. Crabtree, our Consulting Chief Medical Officer, Dr. José Iglesias, and the Chairman of our Medical and Scientific Advisory Board, Dr. Matthias Löhr.”
PharmaCyte’s Consulting Chief Medical Officer, Dr. José Iglesias, stated, “I am pleased that PharmaCyte has submitted its IND to the FDA. The entire PharmaCyte team can now begin work to prepare for the clinical trial. Combining a unique biologic that consists of encapsulated genetically altered human cells with a well-known cancer drug to treat LAPC, PharmaCyte has the possibility of changing the way LAPC is treated in the future. This novel treatment modality may also hold the potential for extending the life span of patients with LAPC.”
The proposed multicenter, randomized, open-label Phase 2b clinical trial is intended to evaluate the efficacy and safety of CypCaps™ (genetically engineered human cells encapsulated using the Cell-in-a-Box® technology) in combination with low doses of the chemotherapy prodrug, ifosfamide, as compared to chemoradiation therapy with capecitabine plus external beam radiation therapy (“EBRT”) or stereotactic body radiation therapy (“SBRT”) alone. The study population will consist of approximately 100 patients. Patients will be randomized in a 1:1 ratio to either treatment with the study therapy or a comparator. The randomization will be stratified by previous treatment (Abraxane® plus gemcitabine or FOLFIRINOX) and the control arm choice (capecitabine/EBRT or SBRT alone).
The primary objective will be determined by progression free survival (“PFS”). The secondary objectives for this study are to determine if CypCaps™ plus low-dose ifosfamide will: (i) increase overall survival (“OS”); (ii) increase objective response rate; (iii) increase the rate of conversion of the pancreatic tumor from inoperable to operable; (iv) decrease the pancreatic cancer tumor marker CA 19-9; and (v) improve a patient’s quality of life. In addition, this clinical trial will assess the safety and tolerability of CypCaps™ plus low dose ifosfamide.
Principal Investigator: Dr Manuel Hidalgo
Chief of the Division of Hematology and Medical Oncology at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center
The E. Hugh Luckey Distinguished Professor of Medicine, Dr. Hidalgo comes to Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center from Beth Israel Deaconess Medical Center in Boston, where he served as chief of the Division of Hematology as well as clinical director of the Rosenberg Clinical Cancer Center. He is also the Theodore W. and Evelyn G. Berenson Professor of Medicine at Harvard Medical School and deputy associate director for clinical sciences at the Dana-Farber/Harvard Cancer Center.
A respected oncologist and premier investigator, Dr. Hidalgo’s work has centered on translational and clinical research in anticancer drug development with a particular emphasis in gastrointestinal cancers. He has developed, tested and helmed the early clinical development of more than 50 new anticancer agents for pancreatic and other solid tumor cancers. Three of those agents, including nab-paclitaxel, are now approved by the U.S. Food and Drug Administration for treatment of GI cancers, including pancreatic cancer. His team also pioneered the development of a personalized cancer model using mice, called mouse avatars, to pre-test cancer treatments.
"Under Dr. Hidalgo’s direction and working with the Joint Clinical
Trials Office at Weill Cornell Medicine and NewYork-Presbyterian/
Weill Cornell Medical Center, the division will enhance its already
robust clinical trials portfolio, which takes the innovative approaches
to cancer therapy from the lab to the patient.
Our priority is ensuring that our clinical trials portfolio is modern,
comprehensive and diverse,” he said, “so patients with advanced
cancers have the opportunity to receive the latest therapies.”
Consulting Chief Medical Officer: José L. Iglesias M.D.
Dr. Iglesias brings a wealth of experience in developing and testing a variety of cancer chemotherapeutic agents to PharmaCyte, including key positions with many prominent biotechnology firms including Eli Lilly, Amgen, Abraxis and Celgene. Dr. Iglesias’s body of work is ideally suited to guide PharmaCyte through its planned Phase 2b clinical trial in LAPC. As the global Vice-President of Clinical Development at Celgene, Dr. Iglesias was the lead physician on the team that obtained FDA approval for Abraxane® (the nab-paclitaxel/gemcitabine combination), which is a first-line therapy in pancreatic cancer.
Chairman and a founder of Austrianova: Prof. Walter H. Günzburg, Ph.D.
The Professor of Virology at the University of Veterinary Medicine, Vienna, Austria and formerly an Adjunct Professor at the National University of Singapore and director of the Christian Doppler Laboratory for Gene Therapeutic Vector Development in Vienna.
Prior to founding Austrianova, he was the Chairman of the board of Austrianova Biotechnology GmbH and Austrianova Biomanufacturing AG. He has also been an advisor to Paktis (Germany) and Tocagen (USA), as well as a founder, board member and former Chairman of Virusure GmbH, a virus testing company located in Vienna, Austria.
He has also served as a member of German Federal Medical Association Ethics Committee for Gene Therapy (Kommission Somatische Gentherapie der Bundesärztekammer) since 1997 as well as a member of the European Academy for the Study of Consequences of Scientific and Technological Advances (since 1998).
Prof. Günzburg has more than 30 years experience in virology, and twenty years experience in cell therapy and bioencapsulation. He has published numerous peer reviewed scientific publications.
Product Manager of Global research for the Biosciences Division of ThermoFisher Scientific: David Judd
David has worked for ThermoFisher for 24 years in cell culture products and applications. David has a broad array of experience in development of media for primary cells and cell lines. He has developed manufacturing processes, cell assays, biochemical analysis, cell culture processes and downstream recovery strategies. He has stepped out of the lab in order to find new ways to provide new workflow solutions for serum products for cell culture applications.
Targeted Chemotherapy
We are employing the Cell-in-a-Box® technology as a platform to develop therapies for solid cancerous tumors using targeted chemotherapy. Our live-cell encapsulation technology-based therapies consist of encapsulating different types of genetically modified living cells depending on the disease being treated. For our leading product candidate, a therapy for pancreatic cancer, about 10,000 genetically modified live cells that produce an enzyme which converts the chemotherapy prodrug ifosfamide into its cancer-killing form are encapsulated in porous, spherical, pinhead-sized capsules, composed largely of cellulose. About 300 of these capsules are placed in the blood supply as close to the tumor in the pancreas as possible. Then about one-third the normal dose of the chemotherapy prodrug ifosfamide is given to the patient intravenously. The prodrug is normally activated in the patient’s liver. By activating the prodrug near the tumor using the Cell-in-a-Box® capsules, our cellular therapy acts as a type of “artificial liver.” Using this “targeted chemotherapy,” we are seeking to create an environment that enables optimal concentrations of the “cancer-killing” form of ifosfamide at the site of the tumor. Because the cancer-killing form of ifosfamide has a short biological half-life, we believe that this approach results in little to no collateral damage to other organs in the body. We also believe this treatment significantly reduces tumor size with no treatment-related side effects.

As shown above, the presence of the capsules in the blood vessel leading to the tumor do not prevent the blood flow to the tumor.

Since the cancer-killing metabolite of ifosfamide has a short half-life, we believe will result in little to no collateral damage to other organs in the body. In an earlier Phase 1/2 clinical trial which used our pancreatic cancer treatment in very sick patients with advanced pancreatic cancer, this targeted chemotherapy not only reduced the tumor size but also resulted in no obvious adverse side effects attributable to the therapy.


Orphan Drug designation from drug regulatory authorities in the U.S. and the E.U.carries with it 7 years of marketing exclusivity in the U.S. and 10 years in the E.U. after the product has been approved for marketing by these regulatory authorities.
Pancreatic Cancer
Pancreatic cancer is a very aggressive cancer with a poor prognosis. It is the third leading cause of cancer-related deaths in the U.S. and the seventh leading cause of cancer-related deaths globally. The five-year survival rate is 8%, reportedly the lowest survival rate of any cancer. It is expected that in 2017 there will be more than 53,000 new cases diagnosed in the U.S. In Europe, the estimate is approximately 80,000. Unfortunately, about 72% of patients will die within the first year of diagnosis. More than 90% will die within two years of diagnosis. Without treatment after diagnosis, patients have a 3-6 month median life expectancy.
Patients with pancreatic cancer are not normally diagnosed until the cancer is advanced and inoperable. There is no cure for pancreatic cancer unless the tumor is surgically removed in its earliest stages of growth. Since the first drug (gemcitabine) was approved in the U.S. for pancreatic cancer in 1996, approximately 40 registrational clinical trials have been conducted. In spite of this, little improvement in median survival time and percentage of one-year survivors has occurred. Most success was achieved when gemcitabine was given in combination with another chemotherapy drug.
The current standard of care for advanced pancreatic cancer is the combination of Abraxane® (a nanoparticle albumin formulation of the drug paclitaxel (Taxol®) plus gemcitabine or the 4-drug combination known as FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin). Abraxane® plus gemcitabine was approved by the U.S. Food and Drug Administration (“FDA”) in September 2013. This combination increased the median survival time by 1.8 months, as compared to gemcitabine alone. It increased the one-year survival time from 22% with gemcitabine alone to 38% with Abraxane® plus gemcitabine. FOLFIRINOX has never been approved by the FDA or any other regulatory agency such as the European Medicines Agency (“EMA”), but was introduced to clinical practice in 2010 due to the results of a Phase 2/3 trial comparing FOLFIRINOX with the single-agent gemcitabine as first-line treatment for patients with metastatic pancreatic cancer. Median overall survival was superior with FOLFIRINOX over gemcitabine. Since the conclusion of that trial, oncologists in Europe began using it as first line therapy. That trend has increased in the U.S. despite the FDA approved combination of Abraxane® plus gencitabine. However, there are severe side effects associated with each chemotherapy combination.
PharmaCyte’s Pancreatic Cancer Therapy
A critical unmet medical need exists for patients with locally advanced, unresectable, non-metastatic pancreatic cancer (“LAPC”) who no longer respond to first-line therapy with either Abraxane® plus gemcitabine or the 4-drug combination known as FOLFIRINOX – the current first-line standards of care for advanced pancreatic cancer. These patients have no effective treatment alternative once their tumors no longer respond to these therapies. Two of the most commonly used treatments are 5-fluorouiracil (“5-FU”) or capecitabine (a prodrug of 5-FU) plus radiation. Both treatments are only marginally effective in treating the tumor and result in serious side effects. Other treatments are being tried in an attempt to address this problem, but their success is far from certain. We are developing a therapy that we believe can serve as a “consolidation therapy” with the current standards of care and address the critical unmet medical need discussed above.
Subject to FDA approval, we plan to commence a clinical trial involving patients with LAPC whose tumors have ceased to respond to either Abraxane® plus gemcitabine or FOLFIRINOX. We had a Pre-Investigational New Drug Application (“Pre-IND”) meeting with the Center for Biologics Evaluation and Research of the FDA (“CBER”) in January 2017. At the Pre-IND meeting, the FDA communicated its agreement with certain aspects of our clinical development plan, charged us with completing numerous tasks and provided us with the guidance on the tasks we need to complete for a successful Investigational New Drug Application (“IND”). The proposed clinical trial is designed to show that our Cell-in-a-Box® plus low-dose ifosfamide therapy can serve as an effective and safe consolidation chemotherapy for patients whose tumors no longer respond after four to six months of therapy with Abraxane® plus gemcitabine or FOLFIRINOX. The trial will initially take place in the United States (“U.S.”) with possible study sites in Europe later in the trial.
In a previous Phase 1/2 open-label, single site 14-patient clinical trial using our therapy and in which only two courses of ifosfamide were given, our treatment was shown to be safe and effective.(See Figure 3). In fact, when the results of that trial were compared to historical data for gemcitabine (the “gold standard” for pancreatic cancer at the time that this trial was conducted) the percentage of one-year survivors was double that previously reported for gemcitabine, the median survival of patients was substantially increased as compared to gemcitabine, and in contrast to gemcitabine, no treatment-related side effects were seen with our therapy. Interestingly, in 3 of the 14 patients, their advanced-stage tumors were reduced in size to the point where they would have been operable.


PharmaCyte’s Trial Design
The trial will be a prospective, multicenter, randomized, open-label Phase 2b study to evaluate the efficacy and safety of CypCapsTM (genetically engineered human cells encapsulated using the Cell-in-a-Box® technology) in combination with low-dose ifosfamide as compared to chemoradiation therapy with capecitabine plus external beam radiation therapy (“EBRT”) or stereotactic body radiation therapy (“SBRT”) alone. The study population will consist of approximately 100 patients. Patients will be randomized in a 1:1 ratio to either treatment with the study therapy or a comparator. The randomization will be stratified by previous treatment (Abraxane® plus gemcitabine or FOLFIRINOX) and the control arm choice (capecitabine/EBRT or SBRT).
The primary objective will be determined by progression free survival (“PFS”). The secondary objectives for this study are to determine if CypCaps plus low-dose ifosfmide will: (i) increase overall survival (“OS”); (ii) increase objective response rate; (iii) increase the rate of conversion from unresectable to resectable pancreatic cancer; (iv) decrease the pancreatic cancer tumor marker CA 19-9; and (v) improve a patient’s quality of life. In addition, this study will assess the safety, tolerability and immunological response of CypCaps plus low-dose ifosfamide.
Breast Cancer
About 1 in 8 U.S. women will develop invasive breast cancer over the course of her lifetime. In 2018, an estimated 266,120 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S., along with 63,960 new cases of non-invasive (in situ) breast cancer. About 2,550 new cases of invasive breast cancer are expected be diagnosed in men. Approximately 40,920 women in the U.S. are expected to die in 2018 from breast cancer, though death rates have been decreasing since 1989. These decreases are thought to be the result of treatment advances, earlier detection through screening and increased awareness. However, for women in the U.S., breast cancer death rates are higher than those for any other cancer besides lung cancer. Worldwide, in 2018 there were over 2 million new cases of breast cancer diagnosed. Also, breast cancer is the most commonly occurring cancer in women throughout the world and the second most common cancer overall.
PharmaCyte Breast Cancer Treatment
According to the American Cancer Society, ten different chemotherapeutic drug combinations have been widely used for years to treat various forms of breast cancer. Nine of these ten combination chemotherapies use the anticancer drug cyclophosphamide (Cytoxan®) as one of the components of the combination.
Cyclophosphamide is a “sister” drug to ifosfamide – the anticancer agent used in the clinical trials in patients with advanced, inoperable pancreatic cancer using PharmaCyte Biotech’s treatment for pancreatic cancer. Both drugs are classified as “prodrugs” and must be activated (converted to their cancer-killing form) for them to be effective. The cytochrome P450 enzyme system in the liver activates both cyclophosphamide and ifosfamide.
For PharmaCyte’s pancreatic cancer treatment, the cells that are encapsulated using the Cell-in-a-Box® technology
contain an isoform (known as CYP2B1) of the cytochrome P450 enzyme system and are efficient at activating ifosfamide. Because of the similarities between ifosfamide and cyclophosphamide, the same encapsulated cells as those used in the pancreatic cancer clinical trials should efficiently and effectively activate cyclophosphamide into its cancer killing form in developing a novel treatment for breast cancer.
Veterinary Phase 1/2 Trial in Dogs with Breast Cancer
To test this hypothesis, Cell-in-a-Box® capsules containing cells with high CYP2B1 activity were used in combination with cyclophosphamide in a veterinary Phase 1/2 veterinary clinical trial in dogs with spontaneously-occurring mammary tumors. This is a good animal model system for testing the effectiveness of cyclophosphamide in treating breast cancer in humans because the development and pathological features of mammary tumors in dogs are very similar to those seen in breast cancer in women. Spontaneous mammary tumors account for about 70% of all cancers in dogs. Usually such tumors are removed surgically, but it has been estimated that more than 55% of dogs develop new cancers after such surgery. Cyclophosphamide was chosen for this veterinary Phase 1/2 trial rather than ifosfamide because it is often used to treat breast cancer in women as well as mammary tumors in dogs. In addition, as noted above, it is a component of most combination chemotherapy regimens used against breast cancer in humans.
A total of 16 dogs were enrolled in the preclinical trial. The dogs were divided into two groups. One group of 6 dogs was treated intravenously with cyclophosphamide alone at a dose of 7 mg/kg of body weight, in four treatments on days 2, 9, 22, and 29. In the 10 other dogs, 20 Cell-in-a-Box® capsules containing the cyclophosphamide-activating cells were implanted at 5 sites around their tumors on day 0 (two days before administration of the first dose of cyclophosphamide) and then cyclophosphamide was administered in the same regimen as the other 6 dogs. Three of the 10 dogs treated with the encapsulated cells/cyclophosphamide combination had two tumors as did one dog treated with cyclophosphamide alone.
As in the pancreatic cancer clinical trials in humans, the capsules and the cells within them were well tolerated – no “safety” issues were seen in the dogs that could be related to the presence of the capsules or to their implantation over the entire 56-day observation period. Although mild side effects from the use of cyclophosphamide were observed in both groups of dogs, these were no more severe than those seen when this drug is used alone to treat mammary cancer in canines.
The final evaluation of the effect of the treatments on tumor size were graded as follows: Complete remission (CR), Partial remission (PR) – regression of the tumor by at least 50% of the initial tumor mass, Stable disease (SD) – regression of the initial tumor mass by less than 50% or its enlargement by less than 25%, Progression of disease (PD) – increase in initial tumor mass of at least 25% or the occurrence of new tumors. Six of the 11 tumors treated with the encapsulated cells/cyclophosphamide combination showed a PR while the other 5 showed SD. Of the 7 tumors in the 6 dogs that received cyclophosphamide alone, 6 tumors showed SD and the other exhibited PD. The median tumor size reduction of the tumors in dogs treated with the combination was 53%. This is in contrast with only a 21% reduction of the size of the tumors in the dogs treated with cyclophosphamide alone.
For one of the dogs with two tumors in the combination-treated group, both tumors showed a similar reduction in size (65% and 62%). In a second dog with two tumors in this group, one tumor that received the combination treatment was reduced in size by 70% while the other tumor, which was exposed to cyclophosphamide alone, was reduced in size by only 14%. The tumors in dogs that were administered cyclophosphamide alone were reduced in size by only about 19%.
The results of this study indicate that the combination of the Cell-in-a-Box® cellulose-based live-cell encapsulation and cyclophosphamide may prove to be of significant benefit not only for the treatment of mammary cancers in the veterinary setting, but also may ultimately find a role in the treatment of breast cancer in humans by optimizing the cancer-killing activity of cyclophosphamide when that drug is used for breast cancer
Live Cell Encapsulation Technology
The Cell-in-a-Box® live cell encapsulation technology employed by PharmaCyte is a “platform” upon which treatments for different diseases may be built. This technology encloses live cells in protective “capsules” about the size of the head of a pin. The capsules are designed to allow blood to enter and nourish the living cells inside them. The live cells thrive while the capsules are in the body. But the capsules are also designed to protect the live cells from attack by the body’s immune system. The live cells are too large to escape from the capsules, and the body’s immune system cells are too large to enter the capsules and destroy them. PharmaCyte is using this technology to develop unique therapies for various forms of cancer and for Type 1 diabetes and insulin dependent Type 2 diabetes.
The encapsulation of living cells using the Cell-in-a-Box® technology is a multi-step process. To be encapsulated, the live cells are first suspended in a medium that contains a proprietary polymer and sodium cellulose sulfate. This suspension is passed through a droplet-forming machine, and the resulting droplets fall into a solution containing another polymer. As the two polymers interact, immediately a membrane forms around each droplet. This develops into a shell around the droplet, resulting in a spherical capsule 0.7-0.8 mm in diameter. For pancreatic cancer, each capsule contains about 10,000 living cells. This number of live cells can differ depending upon the size of the cells encapsulated and for what purpose they are to be used.

Encapsulation Material
The Cell-in-a-Box® capsules have been shown to be safe, effective and durable in humans for approximately two years during two early clinical trials. The capsules are made of bio-inert material (cellulose/cotton). They have pores for nutrient and waste transfer. The pores are too small for the immune system cells to enter or the encapsulated live cells to leave. Also, the live encapsulated cells can be frozen and later thawed with viability of approximately 90% or more upon thawing. Hence, the frozen encapsulated cells have a long-term (5+ years) shelf life which results in manageable logistics.
The capsules produced using the Cell-in-a-Box® encapsulation differ from those produced by others. Our competitors use substances such as alginate (a seaweed derivative) or chitosan, whereas our capsules are made principally of cellulose – a bio-inert material in the human body. The capsules durable, resilient and long-lasting. They can also withstand various external forces unlike those made using other encapsulation methods. The properties of our capsules allows them to be implanted in the human body by using needles or catheters without damage to the human body. Our capsules do not degrade, even after being present in the body for over two years. They do not cause any damage to or inflammation of tissues where they are located in the body.



Latest news...
The FDA’s Center for Biologics Evaluation and Research (CBER) successfully processed PharmaCyte’s DMF
August 5th 2020
PharmaCyte Biotech, Inc. (OTCQB: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that the Drug Master File (DMF) that its partner, Austrianova, filed with the U.S. Food and Drug Administration (FDA) has been accepted without any questions or suggested changes.
The FDA’s Center for Biologics Evaluation and Research (CBER) successfully processed PharmaCyte’s DMF, which provides all confidential and detailed information covering the production of the CypCaps™ final product that was produced by Austrianova and will be used in PharmaCyte’s planned clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, said, "CBER’s acceptance and processing of our Drug Master File without any questions or comments is a credit to the painstaking work and dedication by Austrianova and our consultants to ensuring that the entire process is properly conducted and completed before any further documents are submitted to the FDA.
"We now look forward to presenting the FDA with our Investigational New Drug Application very soon for our planned clinical trial in locally advanced, inoperable pancreatic cancer."
A DMF is submitted to the FDA to provide detailed information about facilities, processes and materials used in the manufacturing, processing and packaging of human drugs. It is a prerequisite to securing approval and commercialization and ensures confidentiality of proprietary information related to the Active Pharmaceutical Ingredient (API). The information contained in a DMF is used to support, among other things, an IND.
The DMF requirements are complex and specific, encompassing every detail involved with the manufacture of the API – from raw materials to analytical methods, process development and optimization. The scrutiny goes all the way back to the starting materials used in the API.
José L. Iglesias M.D. appointed as Consulting Chief Medical Officer (CMO) for its planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
August 10
PharmaCyte Biotech, Inc. (OTCQB: PMCB), a biotechnology company focused on developing targeted cellular therapies for cancer and diabetes using its signature
live-cell encapsulation technology, Cell-in-a-Box®, today announced that it has appointed José L. Iglesias M.D. as Consulting Chief Medical Officer (CMO) for its planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC).
Dr. Iglesias brings a wealth of experience in developing and testing a variety of cancer chemotherapeutic agents to PharmaCyte, including key positions with many prominent biotechnology firms including Eli Lilly, Amgen, Abraxis and Celgene. Dr. Iglesias’s body of work is ideally suited to guide PharmaCyte through its planned Phase 2b clinical trial in LAPC. As the global Vice-President of Clinical Development at Celgene, Dr. Iglesias was the lead physician on the team that obtained FDA approval for Abraxane® (the nab-paclitaxel/gemcitabine combination), which is a first-line therapy in pancreatic cancer.
Dr. Iglesias is also familiar and experienced with the treatment of various abdominal cancers and the use of gemcitabine in patients. He was instrumental in the design of the Phase 3 clinical trial for the development of nab-paclitaxel for use against metastatic pancreatic cancer while at Celgene.
PharmaCyte’s Chief Executive Officer, Kenneth L. Waggoner, stated, “We are extremely fortunate to have Dr. Iglesias join PharmaCyte for our planned Phase 2b clinical trial in LAPC. We interviewed a number of impressive candidates, but his experience in dealing with various cancer chemotherapeutic agents and, more importantly, his direct knowledge in treating pancreatic cancer make him the obvious choice for the position of Consulting CMO for our clinical trial.
I would be remiss in not saying that Dr. Manuel Hidalgo, who will be the Principal Investigator for our planned clinical trial in LAPC, personally recommended Dr. Iglesias to us for consideration. Dr. Hidalgo considers Dr. Iglesias to be one of the most brilliant physicians in the field.”
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
----------------------
LAGUNA HILLS, Calif.–(BUSINESS WIRE)– PharmaCyte Biotech, Inc. (NASDAQ: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced further results from a third test of biocompatibility of its CypCaps™ product candidate for pancreatic cancer. The tests results showed that the empty capsule material is
Read more → LAGUNA HILLS, CA, September 21, 2021 (BUSINESS WIRE) — PharmaCyte Biotech, Inc. (NASDAQ: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today the results of a second U.S. Food and Drug Administration (FDA)-required test of biocompatibility of its CypCaps product for pancreatic
Read more → LAGUNA HILLS, CA, September 16, 2021 (BUSINESS WIRE) — PharmaCyte Biotech, Inc. (NASDAQ: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today the first test results of the biocompatibility studies of its CypCaps™ clinical trial product candidate. These results were from an
Read more → LAGUNA HILLS, CA, September 8, 2021 (BUSINESS WIRE) – PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced that it will be presenting at the H.C. Wainwright 23rd Annual Global Investment Conference to be held
Read more → LAGUNA HILLS, CA, August 31, 2021 (BUSINESS WIRE) — PharmaCyte Biotech, Inc. (NASDAQ: PMCB), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that its clinical trial product, CypCaps™, remains stable and active at 18 months after completing the 18-month timepoint of ongoing
Read more → LAGUNA HILLS, CA, August 25, 2021 (BUSINESS WIRE)—PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced that the Company will expand its product pipeline to again include its diabetes program and its malignant ascites program.
Read more → LAGUNA HILLS, Calif.–(BUSINESS WIRE)– PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced the closing of its previously announced registered direct offering priced at-the-marked under Nasdaq rules, of 14,000,000 shares of the Company’s common stock
Read more → LAGUNA HILLS, CA, August 19, 2021 (BUSINESS WIRE)—PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced that it has entered into definitive agreements with institutional investors for the purchase and sale, in a registered direct
Read more → LAGUNA HILLS, CA, August 16, 2021 (BUSINESS WIRE)—PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today released a statement from Kenneth L. Waggoner, the Chief Executive Officer of PharmaCyte, regarding the Company’s recent listing on Nasdaq
Read more → LAGUNA HILLS, Calif.–(BUSINESS WIRE)– PharmaCyte Biotech, Inc. (NASDAQ: PMCB) (PharmaCyte or Company), a biotechnology company focused on developing cellular therapies for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, today announced the closing of its previously announced underwritten public offering of approximately $15 million. The public offering includes 3,529,412 shares of the Company’s
Read more →























