Replies to post #285520 on Avid Bioservices Inc (CDMO)
02/09/17 9:39 AM







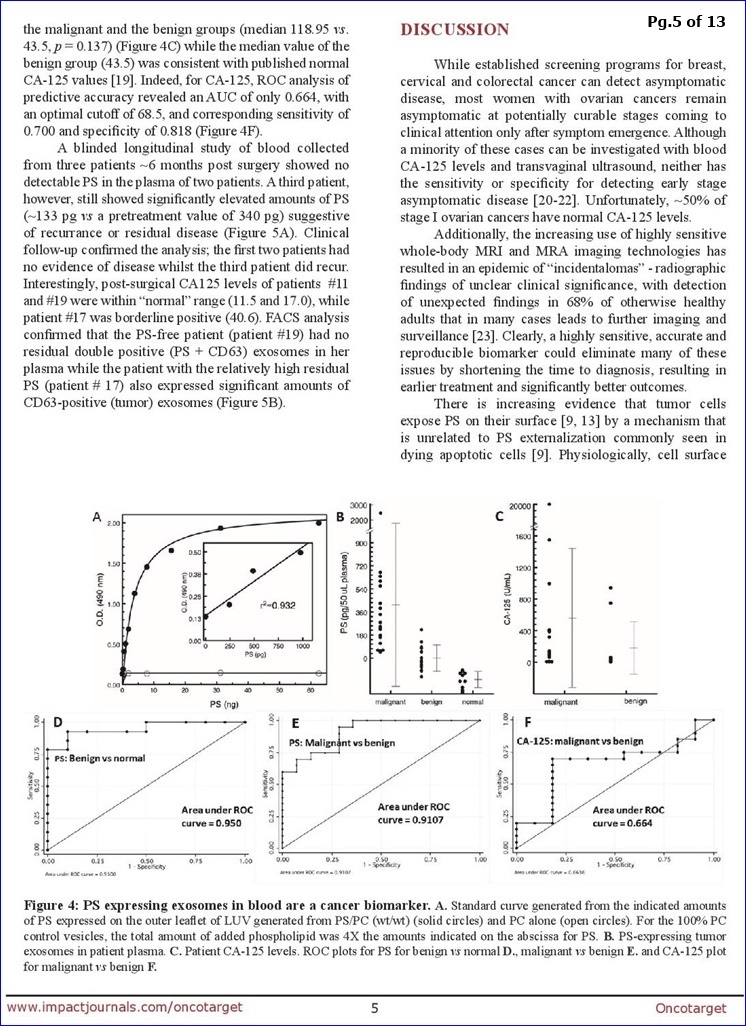




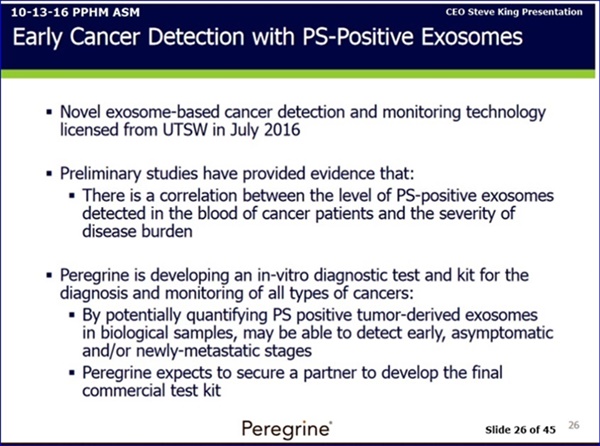



In addition, Alan Schroit, professor of immunology at UT Southwestern, is studying another possible avenue for early detection that's promising so far. In a small study, he worked with Lea, who provided 40 blood samples from her patients with ovarian tumors who were scheduled for diagnostic surgery. Schroit's team performed an ultra-sensitive test for the presence of particles secreted by malignant tumors in the blood samples.
"We were able to predict whether each patient had a benign or malignant tumor, with 100 percent accuracy," Schroit said. After that group of patients underwent surgery or chemotherapy, the same test was performed on a second set of blood samples, and accurately predicted which patients had a recurrence.
"We're confident we can detect early disease, and hopefully, this will turn into a routine screening test," Schroit said. He's working now to launch larger-scale studies.
....
....
https://www.dallasnews.com/life/better-living/2017/12/11/new-hope-early-detection-deadly-ovarian-cancer
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |