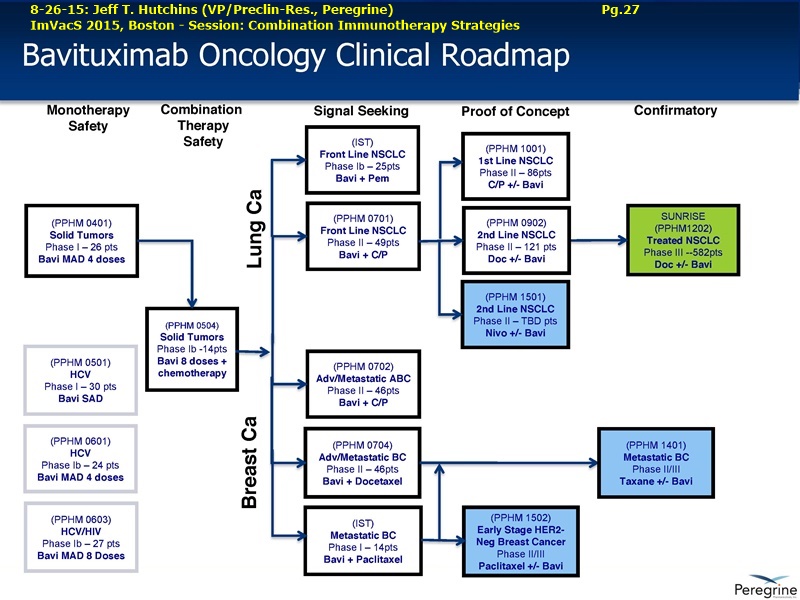
9-10-15/OutsourcingPharma: “Avid Revs Likely to Grow Substantially”
9-10-15: “Peregrine Up on CMO Q1 Sales & Backlog, as New Plant Set to go Online”
By Dan Stanton, Outsourcing-Pharma
http://www.outsourcing-pharma.com/Contract-Manufacturing/Peregrine-up-on-CMO-sales-and-backlog-as-new-plant-set-to-go-online
Manufacturing backlog at Avid Bioservices has reached $42mm as the firm books up space at a new facility currently undergoing its first internal pilot run. For Q1/FY2016, Peregrine Pharmaceuticals reported record revenue from its contact manufacturing business Avid Bioservices of $9.4mm, up 71% year-on-year. But revenues are likely to grow substantially, the firm said, as there is a $42mm committed backlog from existing customers which will be carried-out in part once a new mammalian cell culture manufacturing facility in Tustin, California comes online.
“The new manufacturing suite is fully built and the first internal pilot run is currently underway to verify all systems and equipment are properly functioning,” Peregrine CFO Paul Lytle said during an investor call yesterday. ”Our strategic investment in the Avid Bioservices business is already starting to pay dividends. Our clients are reserving manufacturing slots in the new facility which has increased our revenue backlog to approximately $42mm.”
A large proportion of the firm’s revenues come from its major client, Halozyme Therapeutics, servicing monoclonal antibody development projects with Roche and Baxter. While the company hopes the new facility will attract new customers, it is the current customer base showing the most interest.
“In the new facility, a lot of the interest comes from the existing client base, even as much as we've had new potential customers coming through,” said CEO Steven King. “It's exciting, it's a real nice showpiece and it's really showing in the interest that it's generated from again the existing client base.”
The site, first announced last year, more than doubles Avid’s manufacturing capacity, though some of the space has been reserved to service its parent company’s lead product bavituximab, a chimeric mAb in Phase III trials for non-squamous non-small cell lung cancer. END
• 6-17-15: Avid’s John Haney (ex-Genentech/Pfizer) speaking at BIO-INTL’5/Philly http://tinyurl.com/pnlquu3 & http://tinyurl.com/nl4vbgk
...”Designing & Implementing Avid’s New State-of-the-Art Single-Use Facility for Late Ph.3 & Commercial Prod.” - SK: "We've seen tremendous interest for production in the new facility, both from new & existing clients"
• 12-10-14: Avid to Double Mfg. Capacity (“expanding client roster; potential commercial launch of bavituximab”) http://tinyurl.com/mmc3qgy & http://tinyurl.com/kmdgq8t
• 3-24-15: Avid Receives CMO Leadership Awards for Its Commitment to Innovation & Reliability http://tinyurl.com/psep47f
• 3-12-13: Avid Q3'FY13 GP=$3.3mm; S.King 3-2012, "We have a profitable CMO, Avid Bioservices" http://tinyurl.com/l97rzm8
= = = = = = = = = = = = = = = = = = = = = = = = = = = =
Updated PPHM REVS-BY-QTR TABLE, now thru FY16/Q1 (q/e 7-31-15), per the 7-31-15 10-Q ( http://tinyurl.com/pemub47 ) issued 9-9-15.
• Total Revs since May’06: ($138.6mm/Avid + $24.1mm/Govt + $2.4mm/Lic.) = $165.2mm
• Deferred-Revs at 7-31-15, going fwd into FY’16/Q2 (q/e 10-31-15), total $8.3mm, UP from the $6.6mm of Deferred-Revs at 4-30-15 that drove into FY’16/Q1.
• Avid’s Gross-Profit over last 3 qtrs: $12.5mm on revs of $24.4mm (GP% = 49%)
• Recall, Avid Rev$ from Gov’t DTRA Contract work (6/30/08 – 4/15/11, totaling $24.15mm), went into GOVT-REVS, not AVID-REVS, in the Financials.
Avid’s website: http://www.avidbio.com
AVID PROFITABILITY (GROSS*) BY QTR:
QTR Avid-Rev$ CostofMfg$ Gross-Profit$ GP%
FY13Q1 7-31-12 4,135,000 2,024,000 2,111,000 51%
FY13Q2 10-31-12 6,061,000 3,703,000 2,358,000 39%
FY13Q3 1-31-13 6,961,000 3,651,000 3,310,000 47%
FY13Q4 4-30-13 4,176,000 3,217,000 959,000 23%
FY13 TOTAL: 21,333,000 12,595,000 8,738,000 41%*
FY14Q1 7-31-13 4,581,000 2,670,000 1,911,000 42%
FY14Q2 10-31-13 7,354,000 4,195,000 3,159,000 43%
FY14Q3 1-31-14 3,885,000 2,416,000 1,469,000 38%
FY14Q4 4-30-14 6,474,000 3,829,000 2,645,000 41%
FY14 TOTAL: 22,294,000 13,110,000 9,184,000 41%*
FY15Q1 7-31-14 5,496,000 3,583,000 1,913,000 35%
FY15Q2 10-31-14 6,263,000 4,139,000 2,124,000 34%
FY15Q3 1-31-15 5,677,000 3,113,000 2,564,000 45%
FY15Q4 4-30-15 9,308,000 4,758,000 4,550,000 49%
FY15 TOTAL: 26,744,000 15,393,000 11,151,000 42%*
FY16Q1 7-31-15 9,379,000 4,608,000 4,771,000 51%
*Avid Net-Profit (ie, incl. Selling, G&A) not split out from PPHM-Corp. in the financials.
.
PPHM REVENUES (in thousands) DEFERRED
-------REVENUES------- REVENUES INVEN-
Quarter Avid Govt Lic. TOTAL Avid Govt TORIES
FY07Q1 7-31-06 398 0 23 421 317 0 971
FY07Q2 10-31-06 636 0 48 684 1388 0 1899
FY07Q3 1-31-07 347 0 16 363 2202 0 1325
FY07Q4 4-30-07 2111 0 129 2240 1060 0 1916
FY08Q1 7-31-07 1621 0 4 1625 1820 0 2363
FY08Q2 10-31-07 1863 0 29 1892 1338 0 3500
FY08Q3 1-31-08 1662 0 13 1675 1434 0 2394
FY08Q4 4-30-08 751 0 150 901 2196 0 2900
FY09Q1 7-31-08 1193 324 0 1517 4021 980 4628
FY09Q2 10-31-08 983 958 0 1941 6472 1701 6700
FY09Q3 1-31-09 5778 1048 0 6826 4805 3262 5547
FY09Q4 4-30-09 5009 2683 175 7867 3776 3871 4707
FY10Q1 7-31-09 2070 4671 9 6750 5755 2332 6177
FY10Q2 10-31-09 5308 1510 78 6896 4260 3989 5850
FY10Q3 1-31-10 2945 6854 78 9877 3052 76 3861
FY10Q4 4-30-10 2881 1461 78 4420 2406 78 3123
FY11Q1 7-31-10 983 2111 115 3209 3719 47 4692
FY11Q2 10-31-10 3627 966 78 4671 2447 35 3555
FY11Q3 1-31-11 1922 882 79 2883 4300 40 3915
FY11Q4 4-30-11 1970 681 78 2729 5617 0 5284
FY12Q1 7-31-11 5439 0 216 5655 4145 0 4481
FY12Q2 10-31-11 4154 0 78 4232 2012 0 3178
FY12Q3 1-31-12 3203 0 78 3281 2552 0 2722
FY12Q4 4-30-12 1987 0 78 2065 3651 0 3611
FY13Q1 7-31-12 4135 0 116 4251 6056 0 5744
FY13Q2 10-31-12 6061 0 78 6139 6221 0 5426
FY13Q3 1-31-13 6961 0 78 7039 5061 0 4635
FY13Q4 4-30-13 4176 0 78 4254 4171 0 4339
FY14Q1 7-31-13 4581 0 107 4688 4164 0 5679
FY14Q2 10-31-13 7354 0 0 7354 3468 0 4033
FY14Q3 1-31-14 3885 0 0 3885 4329 0 5224
FY14Q4 4-30-14 6474 0 0 6474 5241 0 5530
FY15Q1 7-31-14 5496 0 0 5496 4670 0 5998
FY15Q2 10-31-14 6263 0 37 6300 3612 0 5379
FY15Q3 1-31-15 5677 0 0 5677 5752 0 6148
FY15Q4 4-30-15 9308 0 0 9308 6630 0 6148
FY16Q1 7-31-15 9379 0 292 9671 8291 0 10457
Totals: 129212 24149 2416 165156 <=since5/1/2006
.
TOTAL REV’s BY YEAR (Avid+Gov’t+Lic):
FY04 4-30-04 3,314 …Avid(CMO)= 3,039 (Avid-Revs don’t incl. Govt-SVCS)
FY05 4-30-05 4,959 …Avid(CMO)= 4,684
FY06 4-30-06 3,193 …Avid(CMO)= 3,005
FY07 4-30-07 3,708 …Avid(CMO)= 3,492
FY08 4-30-08 6,093 …Avid(CMO)= 5,897
FY09 4-30-09 18,151 …Avid(CMO)= 12,963
FY10 4-30-10 27,943 …Avid(CMO)= 13,204
FY11 4-30-11 13,492 …Avid(CMO)= 8,502
FY12 4-30-12 15,233 …Avid(CMO)= 14,783
FY13 4-30-13 21,683 …Avid(CMO)= 21,333
FY14 4-30-14 22,401 …Avid(CMO)= 22,294
FY15 4-30-15 26,781 …Avid(CMO)= 26,744
...Total Gov’t Revs from 7-2008 inception thru FY11Q1(Apr’11): $24.15mm