| Followers | 449 |
| Posts | 41478 |
| Boards Moderated | 5 |
| Alias Born | 09/26/2009 |
Saturday, November 17, 2012 10:01:58 AM
EPGL WEEKEND DD Sheet $$$$$ MUST SEE $$$$$

Information in this DD sheet was put together by Vilingzskillz and has been gathered from sources including http://www.epglmed.com. Information in this DD sheet should be used as a reference only and up to you to make a sound investment decision. For me i truely believe this is a buy and hold
EP Global Communications, Inc. (ticker EPGL) will change its name to EPGL Medical Sciences, Inc. in early 2013. EPGL Medical Sciences, Inc. is a new biomedical device manufacturing and marketing company. The Company is focused on developing and marketing medical devices for both diagnosis and treatment of chronic pain. EPGL Med’s first medical device, the MPDD, will be released in 2013 to awaiting physicians worldwide.
The FDA cleared MPDD medical device can help physicians detect the exact point of pain origination in muscles as never before. The Company is currently working to invent and develop additional cutting edge medical device technology products to introduce subsequently to the MPDD, including biomedical devices which utilize Bio-Micro-Electro Mechanical Systems technology (Bio-MEMS). Bio-MEMS is one of the fastest growing and promising new frontiers in medical technology today with possibilities in both medical diagnostic and treatment applications.

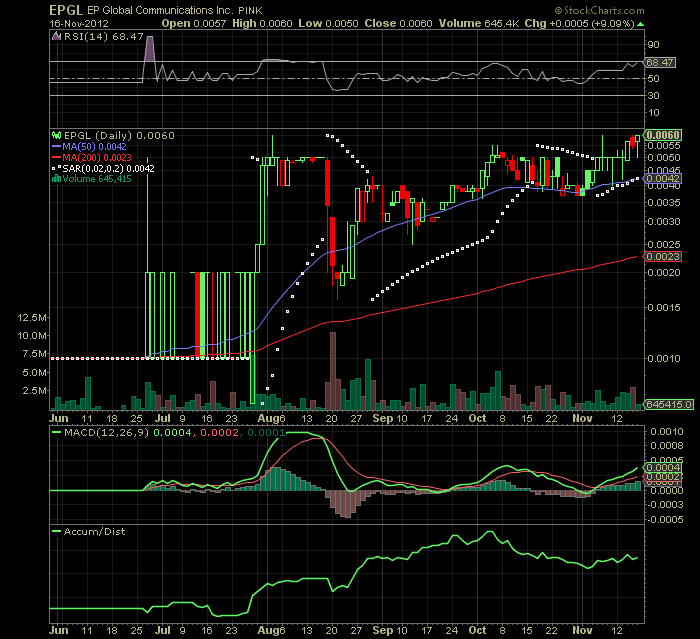
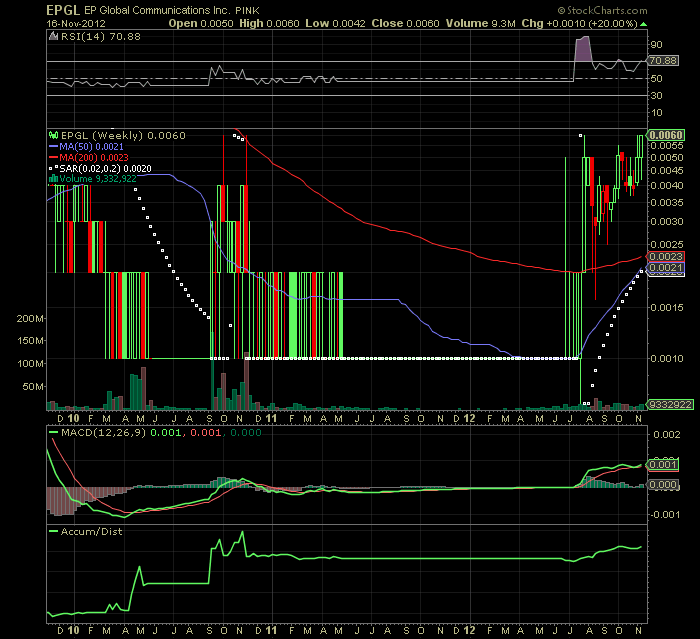
TICKER: EPGL
LAST: .006
52 WEEK TRADING RANGE: .0007 -.006
SENTIMENT: STONG BUY "VIKINGZSKILLZ OPINION only"
FOLLOW EPGL on twitter, worth looking through the last month of tweets to see where comany is going. Can view at this link
http://www.epglmed.com/EPGL_Medical_Sciences/Home.html
1 YEAR DAILY CHART:
1 YEAR WEEKLY CHART
EPGL VIDEOS >>>>MUST SEE!!!!!
Alisyn Camerota, the host of Fox News' 'Fox & Friends 1st', reports on a new and very promising, nonsurgical treatment for severe back pain.
Harry Smith spoke with Dr. Norman Marcus about tips for preventing and easing back pain.
A film clip from ABC's Good Morning America, showing how the Muscle Pain Detection Device changed the life of two patients who were totally disabled by persistent back and leg pain and had been considered untreatable.
A clip from NBC's Today Show with Dr. Norman Marcus and a pain-free patient discussing how back pain can often be cured without surgery or mind-altering medications
EPGL TEAM ---->>>>> VERY IMPRESSIVE
Company Medical Director - Corey W. Hunter M.D
Corey W. Hunter, MD is based in New York City. He completed his residency in physical medicine and rehabilitation at NYU Langone Medical Center and a fellowship in pain medicine at Weill Cornell Medical College. Dr. Hunter's specialties include pain diagnosis and treatment, disorders of the spine and peripheral nervous system, with a special interest in advanced interventional techniques and minimally invasive spinal procedures. Dr. Hunter was pivotal in conducting early research on the MPDD device. Along with NYU physicians, Michel Dubois M.D. and Shengping Zou M.D., Dr. Hunter headed the team which published one of the first research studies showing significant improvement for the detection of pain caused by muscles, using the MPDD device versus traditional manual pressure (MP) for the diagnosis and treatment Myofascial Pain Syndrome which can include chronic back pain, neck pain, migraine, fybromyalgia and more. These findings greatly contributed to the early progress of the MPDD development and its subsequent FDA 510k clearance and patent awards.
Eric Lee M.D.M.A.
Eric Lee M.D. M.A. graduated from Yale University and completed medical school at Boston University. He completed his residency in Physical Medicine and Rehabilitation at NYU Langone Medical Center where he has continued with a Pain Medicine fellowship. Dr. Lee is intimately familiar with the MPDD device and used it as a diagnostic tool in a study of using advanced techniques to treat myofascial pain along with Dr. Michel Dubois MD at NYU. Dr. Lee's professional interests include pain diagnosis and treatment, disorders of the spine, central, and peripheral nervous system, with a special interest in advanced interventional techniques and minimally invasive spinal procedures. Dr. Lee will oversee further additions to the EPGL Medical management team and advancement of new medical devices technologies the Company has interest in pursuing.
David T. Markus Ph.D.
David T. Markus holds a Ph.D. in Biomedical Engineering and a MS in Electrical Engineering with an emphasis in MEMS Microelectronics and Biomedical. Dr. Markus spent 11 years with Raytheon and holds 8 US patents and has 9 other US Patents Pending. He has been involved in research for several of the world's leading technological institutions, including the Office of Naval Research in Arlington, Virginia, NASA Jet Propulsion Laboratory in Pasadena, CA and he has been published 15 times for various technical conferences. He was a principal investigator on SBIR DARPA Phase I and Phase II, and "Ultraflexible Substrate" for Macroelectronics Program by Dr. Robert Reuss at DARPA. He has been involved in the engineering and the development on seven surgical devices, including devices for Cataract surgery, Intra-Ocular Lens Delivery, Arthroscopy, Endodontic Endoscope, Micro Endoscope and Neural Electrodes. Additionally he was instrumental on developing three medical laboratory devices, including for In-Vitro Fertilization, PCR instrumentation and drug discovery. Finally, Dr. Markus is fluent in English, Chinese-Madarin, Taiwanese-FuJian and Indonesian languages. A full bio for Dr. Markus will be made available on the Company website in the near future.
Ryan M. Stellar M.E. B.E.
EPGL is proud to have Ryan M. Stellar M.E. B.E. as part of the team. Mr. Stellar is a highly accomplished biomedical engineer who was intimately involved with the creation of the MPDD device as the Chief Engineer during its creation at Stevens Proof of Concept (SPOC). Mr. Stellar graduated from Stevens Institute of Technology in 2006 with a degree in Biomedical Engineering and a Minor in Economics. Mr. Stellar has been with Medtronic, Inc. for six years prior to leaving this year. While at Medtronic, among many other accomplishments, he successfully directed global launches of two portfolio critical products in the cardiac rhythm device market: DF4 Lead Connector System & CareLink Network for Heart Failure. Mr. Stellar is an expert in medical device manufacturing resources and distribution channels as well as customer relationship management.
Interim Company President & CEO - Michael Hayes
Michael Hayes was President of Digital Health Sciences, Inc. prior to that Company being acquired by EPGL in July 2012. Mr. Hayes has presided over the debt restructuring of EP Global Communications, Inc. along with Pricewaterhouse Coopers since early 2012. Mr. Hayes is now in charge of assembling a new management team for EPGL, including all Company management, medical and scientific personnel going forward. Mr. Hayes has made it a singular priority to bring aboard only the world’s top professionals in the field of medical sciences to EPGL and thereby build the Company into a major player in the medical device industry over the next several years. Mr. Hayes has also committed to prioritizing shareholders and creating significant value for their investments over time.
IR/PR Manager Mr. Brady Peterson
Brady Peterson was recently named Manager of Investor Relations and Public Relations as well as Information Technologies for EPGL Med. Mr. Peterson is the consummate professional who brings 15 years of Customer Relations and marketing experience to EPGL. The EPGL team is pleased to have him aboard and we believe customers and investors alike will appreciate his professionalism.
Company Vice President
Pending TBA
Company CFO
Pending TBA
Company Board of Directors
Pending TBA
Published Research
A New Muscle Pain Detection Device to Diagnose Muscles as a Source of Back and/or Neck Pain
Corey Hunter, MD,* Michel Dubois, MD,† Shengping Zou, MD,† William Oswald, PT,‡ Kathleen Coakley,§ Mourad Shehebar,¶ and Ann Marie Conlon, RN†
ABSTRACT
Background. Trigger point (TrPs) identification has become the mainstay of diagnosis for the treatment of Myofascial Pain Syndrome; however, manual pressure (MP) to identify TrPs by determining low-pressure pain threshold has low interrater reliability and may lack validity since it is done on inactive muscles. To elicit contractions and mimic an active muscle or movement that “causes” pain, a Muscle Pain Detection Device (MPDD) has been developed. A selected muscle is stimulated and painful muscles are precisely detected, allowing distinctions between primary and referred muscle pain as well as distinguishing other functional muscle pain thought to cause MPS.
RECENT PR's
EP Global Communications, Inc. Lands One of the World's Leading Biomedical Engineers
Mon, Sep 24, 2012 7:33 AM EDT
http://finance.yahoo.com/news/ep-global-communications-inc-lands-113335036.html
EP Global Communications, Inc., Announces First Orders for MPDD Medical Device and the Addition of Eric Lee M.D. M.A. to Medical Team
IRVINE, Calif., Aug 30, 2012
http://www.marketwatch.com/story/ep-global-communications-inc-announces-first-orders-for-mpdd-medical-device-and-the-addition-of-eric-lee-md-ma-to-medical-team-2012-08-30

Information in this DD sheet was put together by Vilingzskillz and has been gathered from sources including http://www.epglmed.com. Information in this DD sheet should be used as a reference only and up to you to make a sound investment decision. For me i truely believe this is a buy and hold
EP Global Communications, Inc. (ticker EPGL) will change its name to EPGL Medical Sciences, Inc. in early 2013. EPGL Medical Sciences, Inc. is a new biomedical device manufacturing and marketing company. The Company is focused on developing and marketing medical devices for both diagnosis and treatment of chronic pain. EPGL Med’s first medical device, the MPDD, will be released in 2013 to awaiting physicians worldwide.
The FDA cleared MPDD medical device can help physicians detect the exact point of pain origination in muscles as never before. The Company is currently working to invent and develop additional cutting edge medical device technology products to introduce subsequently to the MPDD, including biomedical devices which utilize Bio-Micro-Electro Mechanical Systems technology (Bio-MEMS). Bio-MEMS is one of the fastest growing and promising new frontiers in medical technology today with possibilities in both medical diagnostic and treatment applications.

TICKER: EPGL
LAST: .006
52 WEEK TRADING RANGE: .0007 -.006
SENTIMENT: STONG BUY "VIKINGZSKILLZ OPINION only"
FOLLOW EPGL on twitter, worth looking through the last month of tweets to see where comany is going. Can view at this link
http://www.epglmed.com/EPGL_Medical_Sciences/Home.html
1 YEAR DAILY CHART:

1 YEAR WEEKLY CHART

EPGL VIDEOS >>>>MUST SEE!!!!!
Alisyn Camerota, the host of Fox News' 'Fox & Friends 1st', reports on a new and very promising, nonsurgical treatment for severe back pain.
Harry Smith spoke with Dr. Norman Marcus about tips for preventing and easing back pain.
A film clip from ABC's Good Morning America, showing how the Muscle Pain Detection Device changed the life of two patients who were totally disabled by persistent back and leg pain and had been considered untreatable.
A clip from NBC's Today Show with Dr. Norman Marcus and a pain-free patient discussing how back pain can often be cured without surgery or mind-altering medications
EPGL TEAM ---->>>>> VERY IMPRESSIVE
Company Medical Director - Corey W. Hunter M.D
Corey W. Hunter, MD is based in New York City. He completed his residency in physical medicine and rehabilitation at NYU Langone Medical Center and a fellowship in pain medicine at Weill Cornell Medical College. Dr. Hunter's specialties include pain diagnosis and treatment, disorders of the spine and peripheral nervous system, with a special interest in advanced interventional techniques and minimally invasive spinal procedures. Dr. Hunter was pivotal in conducting early research on the MPDD device. Along with NYU physicians, Michel Dubois M.D. and Shengping Zou M.D., Dr. Hunter headed the team which published one of the first research studies showing significant improvement for the detection of pain caused by muscles, using the MPDD device versus traditional manual pressure (MP) for the diagnosis and treatment Myofascial Pain Syndrome which can include chronic back pain, neck pain, migraine, fybromyalgia and more. These findings greatly contributed to the early progress of the MPDD development and its subsequent FDA 510k clearance and patent awards.
Eric Lee M.D.M.A.
Eric Lee M.D. M.A. graduated from Yale University and completed medical school at Boston University. He completed his residency in Physical Medicine and Rehabilitation at NYU Langone Medical Center where he has continued with a Pain Medicine fellowship. Dr. Lee is intimately familiar with the MPDD device and used it as a diagnostic tool in a study of using advanced techniques to treat myofascial pain along with Dr. Michel Dubois MD at NYU. Dr. Lee's professional interests include pain diagnosis and treatment, disorders of the spine, central, and peripheral nervous system, with a special interest in advanced interventional techniques and minimally invasive spinal procedures. Dr. Lee will oversee further additions to the EPGL Medical management team and advancement of new medical devices technologies the Company has interest in pursuing.
David T. Markus Ph.D.
David T. Markus holds a Ph.D. in Biomedical Engineering and a MS in Electrical Engineering with an emphasis in MEMS Microelectronics and Biomedical. Dr. Markus spent 11 years with Raytheon and holds 8 US patents and has 9 other US Patents Pending. He has been involved in research for several of the world's leading technological institutions, including the Office of Naval Research in Arlington, Virginia, NASA Jet Propulsion Laboratory in Pasadena, CA and he has been published 15 times for various technical conferences. He was a principal investigator on SBIR DARPA Phase I and Phase II, and "Ultraflexible Substrate" for Macroelectronics Program by Dr. Robert Reuss at DARPA. He has been involved in the engineering and the development on seven surgical devices, including devices for Cataract surgery, Intra-Ocular Lens Delivery, Arthroscopy, Endodontic Endoscope, Micro Endoscope and Neural Electrodes. Additionally he was instrumental on developing three medical laboratory devices, including for In-Vitro Fertilization, PCR instrumentation and drug discovery. Finally, Dr. Markus is fluent in English, Chinese-Madarin, Taiwanese-FuJian and Indonesian languages. A full bio for Dr. Markus will be made available on the Company website in the near future.
Ryan M. Stellar M.E. B.E.
EPGL is proud to have Ryan M. Stellar M.E. B.E. as part of the team. Mr. Stellar is a highly accomplished biomedical engineer who was intimately involved with the creation of the MPDD device as the Chief Engineer during its creation at Stevens Proof of Concept (SPOC). Mr. Stellar graduated from Stevens Institute of Technology in 2006 with a degree in Biomedical Engineering and a Minor in Economics. Mr. Stellar has been with Medtronic, Inc. for six years prior to leaving this year. While at Medtronic, among many other accomplishments, he successfully directed global launches of two portfolio critical products in the cardiac rhythm device market: DF4 Lead Connector System & CareLink Network for Heart Failure. Mr. Stellar is an expert in medical device manufacturing resources and distribution channels as well as customer relationship management.
Interim Company President & CEO - Michael Hayes
Michael Hayes was President of Digital Health Sciences, Inc. prior to that Company being acquired by EPGL in July 2012. Mr. Hayes has presided over the debt restructuring of EP Global Communications, Inc. along with Pricewaterhouse Coopers since early 2012. Mr. Hayes is now in charge of assembling a new management team for EPGL, including all Company management, medical and scientific personnel going forward. Mr. Hayes has made it a singular priority to bring aboard only the world’s top professionals in the field of medical sciences to EPGL and thereby build the Company into a major player in the medical device industry over the next several years. Mr. Hayes has also committed to prioritizing shareholders and creating significant value for their investments over time.
IR/PR Manager Mr. Brady Peterson
Brady Peterson was recently named Manager of Investor Relations and Public Relations as well as Information Technologies for EPGL Med. Mr. Peterson is the consummate professional who brings 15 years of Customer Relations and marketing experience to EPGL. The EPGL team is pleased to have him aboard and we believe customers and investors alike will appreciate his professionalism.
Company Vice President
Pending TBA
Company CFO
Pending TBA
Company Board of Directors
Pending TBA
Published Research
A New Muscle Pain Detection Device to Diagnose Muscles as a Source of Back and/or Neck Pain
Corey Hunter, MD,* Michel Dubois, MD,† Shengping Zou, MD,† William Oswald, PT,‡ Kathleen Coakley,§ Mourad Shehebar,¶ and Ann Marie Conlon, RN†
ABSTRACT
Background. Trigger point (TrPs) identification has become the mainstay of diagnosis for the treatment of Myofascial Pain Syndrome; however, manual pressure (MP) to identify TrPs by determining low-pressure pain threshold has low interrater reliability and may lack validity since it is done on inactive muscles. To elicit contractions and mimic an active muscle or movement that “causes” pain, a Muscle Pain Detection Device (MPDD) has been developed. A selected muscle is stimulated and painful muscles are precisely detected, allowing distinctions between primary and referred muscle pain as well as distinguishing other functional muscle pain thought to cause MPS.
RECENT PR's
EP Global Communications, Inc. Lands One of the World's Leading Biomedical Engineers
Mon, Sep 24, 2012 7:33 AM EDT
http://finance.yahoo.com/news/ep-global-communications-inc-lands-113335036.html
EP Global Communications, Inc., Announces First Orders for MPDD Medical Device and the Addition of Eric Lee M.D. M.A. to Medical Team
IRVINE, Calif., Aug 30, 2012
http://www.marketwatch.com/story/ep-global-communications-inc-announces-first-orders-for-mpdd-medical-device-and-the-addition-of-eric-lee-md-ma-to-medical-team-2012-08-30
Stop by and follow the STOCK PLAYAS Board, alot of great stock calls and always fun!

http://investorshub.advfn.com/%C2%BB%C2%BB%C2%BB-Stock-Playas-%C2
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.









