Tuesday, July 31, 2012 5:29:07 PM
"Diamond in the Rough"--VYCO--Complete DD Page:
You wanna make money right? Well time = money. So take the time to sit down, read this post. You will be impressed at the end at this legit company.
Non-penny penny stock with actual FDA approved, effective products
$100+ million dollar potential here
Company: Vycor Medical Inc.
Vycor Medical, Inc. is a medical device company committed to making neurological brain, spinal and other surgical procedures safer and more effective.
Vycor's innovative medical instruments are designed to optimize neurosurgical site access, reduce patient risk, accelerate recovery, and add tangible value to the professional medical community.
So basically, Vycor Medical was founded on this basic challenge: "There must be a better way". A better way to access surgical locations without unduly damaging surrounding tissue. A less invasive means to perform critical procedures, so that collateral trauma can be minimized and postoperative recovery accelerated. And a better breed of surgical access instrumentation for enhancing performance, safety and results such as the ViewSite Brain Access System, our premiere product line.
Operates in two complementary business units:
1. Vycor's ViewSite Brain Access System (VBAS), a next generation brain access system, is being used in brain surgeries in the US and internationally. The company is ISO 13485:2003 compliant, has U.S. FDA 510(k) clearance for brain and spine surgeries, CE Marking for the European Market and Canadian HPB licensing for brain, and has SFDA approval in China and most recently also regulatory approval in Japan.
The common instruments used in brain retraction procedures are ribbon or blade retractors (might I add VBAS costs less) that assist in the performance of some intraventricular and sub cortical procedures. Even though these instruments are still in use, neurosurgeons have a need for devices that provide better surgical outcomes – results that are beneficial to both the surgeon and the patient.
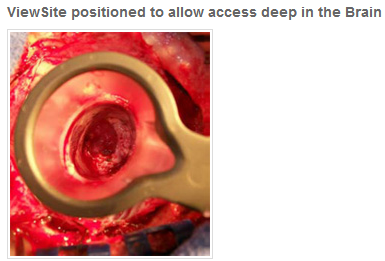
ViewSite™ Brain Access System is a revolutionary approach in brain retraction. Each ViewSite system consists of an introducer and a working channel port that allows the surgeon a seamless entry to the targeted site while distributing brain tissue evenly in a 360° dispersion pattern.
Other ViewSite benefits include superior binocular vision to see in and around the surgical site; multiple sizes in different widths and lengths to meet all surgical needs and compatibility with most surgical arms to avoid accidental displacement or movement during surgery.
--Cost-effective (shorter operating time, reduced post-operative recovery time)
--More efficient
--Less invasive (less damage to surrounding tissue)
--More accurate target access
--FDA approved
--Little to no competition
--Patents [#1 in Russia, #2 in China, 13 patents pending for its Brain and Spine technology and products in: the U.S. (3), Canada (2), Europe (2), India (2), Japan (2), and Hong Kong (2)]


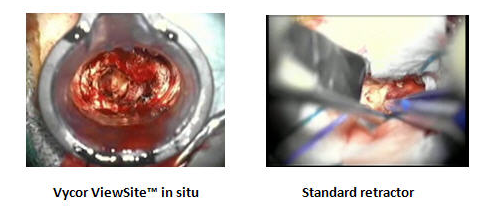
VBAS vs. Standard Retractors
To give you a clearer idea on the effectiveness of VBAS, read below.
The goal of safely accessing the surgical site and creating a clear and stationary working channel to perform delicate procedures is what the ViewSite is all about. There is no pushing on the surgical site numerous times to see the selected area and no clutter of instrumentation during the procedure.
The ample working channel allows for multiple instruments to be used simultaneously while providing an excellent depth of view of the site as well as the brain wall. The working channel comes in four widths – 12mm, 17mm, 21mm and 28mm to meet your exact requirements.
Wanna see VBAS in action?
Video #1: http://vimeo.com/24741351
Video #2: http://vimeo.com/vycormedical/bulletcase
More info: VBAS Outlined by Dr. Quinones-Hinojosa, M.D.
How about Konstantin V. Slavin, MD?
Or Donald M. O'Rourke, MD, Ezriel E. Kornel, MD F.A.C.S, David J. Langer, MD, Ramin Rak, MD, Henry H. Woo, MD, Michael W. Weaver, MD, or John Ragheb, MD?
All have positive reviews of VBAS in the link below:
http://www.vycormedical.com/products/testimonials.html

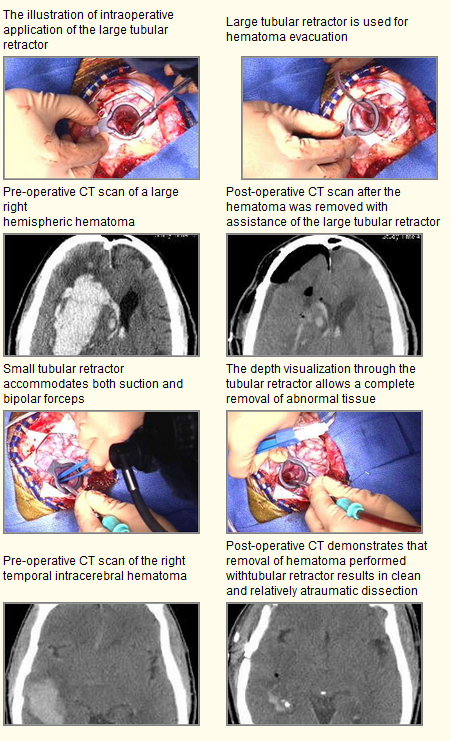
There have been many successful surgeries performed with the ViewSite™ Brain Access System. Below is a listing* that includes these surgeries along with website information regarding clinical information on these surgeries:
Intracerebral Hematomas
www.nyp.org/health/cerebral-contusion-intracerebral-hematoma.html
Arterio-Venous Malformation (AVM)
www.ninds.nih.gov/disorders/avms/avms.htm
Cavernous Malformations
www.ninds.nih.gov/disorders/cavernous_malformation/cavernous_malformation.htm
Metastatic and Primary Brain Tumors (including frontal tumor resection)www.abta.org/siteFiles/SitePages/5E8399DBEEA8F53CBBBBF212C63AE113.pdf
Cerebral Cysts
www.abta.org/sitefiles/sitePages/4F77F57A14D6EBBE5C3FBC447B78FD57.pdf
Intraaxial Tumors and Lesions
www.ninds.nih.gov/find_people/groups/brain_tumor_prg/intraaxial.htm
Intraventricular Tumors
www.nyp.org/health/neuro_intraventricular.html
*It is important to note that the surgeries listed above are examples of applicable cases the ViewSite device can be used in. The ViewSite is not limited to these surgeries.
Not enough?
Well here are three clinical reports on VBAS.
Minimally Invasive Trans-Portal Resection ofDeep Intracranial Lesions by S. M. Raza, P. F. Recinos, J. Avendano, H. Adams, G. I. Jallo, and A. Quinones-Hinojosa
http://www.vycormedical.com/products/Journal%20of%20Minimally%20Invasive%20Neurosurgery%20Web.pdf
Vycor ViewSite TC®: Endoscope-guided Intraparenchimal Brain Tumor Resection
http://www.vycormedical.com/products/Prevedello%20case%201.pdf
Vycor ViewSite TC®: Endoscopic Intraparenchimal Brain Tumor Resection with Image Guidance
http://www.vycormedical.com/products/Dr%20Prevedello%20Case%202.pdf
So we’ve established that the product is top-of-the-line-amazing. Now, let’s talk about the distributors. The more the merrier. More distributors = more marketing = more revenue.
1. USA
2. Australia
3. China
4. Greece
5. Italy
6. Japan
7. Some of Germany
8. South Korea
9. Spain
10. Sweden
11. Switzerland
12. United Kingdom
13. Filed for registration in Russia
14. The Benelux (Belgium, Netherlands, and Luxembourg)
Any questions? Check the Frequently Asked Questions page:
http://www.vycormedical.com/products/faq.html
Not on there?
Vycor Medical Inc
6401 Congress Ave, Suite 140
Boca Raton, FL 33487
Phone: 561-558-2020
fax 631.794.2444
But wait, we’re not done. VBAS is not the online product Vycor produces. Their next, FDA-approved top-of-the-line-amazing product is:

2. NovaVision provides a home-based treatment of vision rehabilitation after stroke or brain injury called Vision Restoration Therapy (VRT™). TVRT is an FDA-cleared, patented, non-invasive medical device that enhances visual functions in patients with impairments due to stroke, surgical intervention, and traumatic brain injury (TBI). Some of the most common types of vision impairments that NovaVision VRT can treat include, Hemianopia, Scotoma, Quadrantanopia, and Amblyopia.
VRT is FDA 510(k) cleared and has CE Marking in Europe as a Class 1 device. It is the only FDA 501(k) cleared medical device in the U.S. aimed at the restoration of vision for neurologically induced vision loss.
While speech, physical, and occupational therapies are the long-standing treatment standards for stroke and TBI survivors, VRT is the first FDA-cleared clinical component of vision rehabilitation to physically enhance the visual field after a stroke or TBI.

Explanation video:
http://vimeo.com/39765566
The time between the injury and beginning therapy is not significant. There have been many successful outcomes for patients who suffered vision loss decades before receiving the therapy. One such case was that of a World War II veteran who benefited from VRT.
Soooooo how successful is VRT?
88% of VRT patients surveyed have mentioned at least one significant benefit as a result of performing VRT, such as improved reading, mobility, and in a few cases, driving again. A retrospective study of 161 patients from 16 medical centers across the US demonstrated that 76% of patients who completed this type of vision rehabilitation showed measurable improvements in their vision fields.
Other studies have produced similar results. As with all therapies individual results vary, and there is no way to predict how you will respond to VRT.
Clinical data have shown that the average vision gain from VRT vision rehabilitation is a five degree shift in the border of the blind field, which can have an exponential impact on a patient’s daily life. A 5 degree shift in the border of the blind field often leads to better reading, better mobility, less collisions, among other effects.
For the majority of patients, the improvement is not sufficient to return to driving, however, quite a few patients have achieved this goal. 88% of patients mention a significant improvement in quality of life.
Results do not appear to be age or gender dependent.
Now read some VRT Patient Testimonials:
http://www.novavision.com/images/stories/PDF/vrt-patient-grid-booklet.pdf
Dr. Michael Rosenberg, Neuro-Ophthalmologist administering VRT at New Jersey Neuroscience Institute at JFK Medical Center states that previously there were only ways to help stroke patients with vision loss adapt to their problem, and VRT actually addresses the problem.
VRT in action:
NovaVision Advisory Board:
1. Alvaro Pascual-Leone, MD, PhD, is Professor of Neurology at Harvard Medical School; Director of the Berenson-Allen Center for Noninvasive Brain Stimulation; Program Director of the Harvard-Thorndike Clinical Research Unit; and an Attending Neurologist at Beth Israel Deaconess Medical Center — all in Boston. He is a practicing behavioral neurologist and movement disorders specialist.
2. Jason J S Barton, MD PhD FRCPC - He is a neurologist, neuro-ophthalmologist and neuroscientist in visual cognition. He obtained his MD and his neurology residency at the University of British Columbia, and then did a clinical neuro-ophthalmology fellowship at the University of Iowa, followed by a PhD at the University of Toronto with Jim Sharpe.
3. Jose G. Romano, MD - Dr. Romano is an Associate Professor of Clinical Neurology, the Director of the Cerebrovascular Division at the University of Miami Miller School of Medicine and the Director of the Stroke Team at Jackson Memorial Hospital in Miami, Florida.
Acquisition of Sight Science, Ltd.
On January 4, 2012, NovaVision, entered into various agreements with Professor Arash Sahraie ("Prof. Sahraie") and the University of Aberdeen (Scotland) (the "University"), relating to the acquisition of all of the shares of Sight Science, Ltd. ("Sight Science") by NovaVision.
Sight Science was established in 2009 based on the research of Professor Arash Sahraie at the University of Aberdeen. Sight Science, which is owned jointly by Prof. Sahraie and the University of Aberdeen, provides an interactive computer-based therapy called Neuro-Eye Therapy ("NeET"), which patients administer at home.
To date, over 100 patients have utilized NeET. The Company has 5 patents granted in the UK, France, Germany, Switzerland and Singapore and 2 patents pending in the U.S. and Canada. Prof. Sahraie has conducted extensive research on blindsight and residual visual processing after brain injury, and is highly regarded in the field.
As part of the transaction, Prof. Sahraie agreed to join NovaVision as its Chief Scientific Officeron a part-time secondment basis from the University for a minimum of 5 years. Prof. Sahraie is responsible for driving NovaVision's scientific effort to develop and validate technologies in vision rehabilitation for visual field defects resulting from brain injury.
The acquisition also created a long-term relationship between the Company, NovaVision and the University, which is a leading medical research center. The University also entered into a Patent Agreement and Assignation Agreement with Sight Science where all of the intellectual property held by the University and which was related to Sight Science's business was assigned to Sight Science.
Both NovaVision's VRT and Sight Science's NeET work on the basis that repeated stimulation of the blind areas by either bright patches of light (VRT) or the specific spatial patterns (NeET) and can lead to increases in sensitivity of the blind areas. Patients progress after VRT appears to be initiated at the blind and sighted borders whereas NeET results in changes deep within the field damage. Both therapies are able to demonstrate improvements in both visual sensitivity and activities of daily living. The Company believes that these therapies are highly complementary.
--Niche market
--Little to no competition
--Proved (over 15 year of clinical research)
--Effective (in approximately 70% of cases patients using VRT system over a three to six month period experience significant recovery in at least one functional outcome -- the improvement conferred by VRT is permanent)
--Patents [ 23 granted patents are in the U.S. (11), Canada (2), Europe (4), Australia (1), China (2), Hong Kong (1), India (1) and Japan (1).
--The Company's 15 pending patents are in the U.S. and Canada (5), Europe (5), Australia (2), and Japan (3)]
--NovaVision substantial potential market: $2.6bn in US, over $20bn globally
--Reviewed and accepted by the scientific community
Revenue Growth:
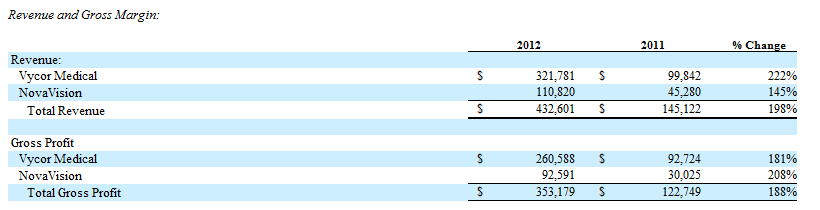

More information:
Research report by Harbinger Research:
http://a.eqcdn.com/harbingerresearch/media/b767cf61ff1d00bcf70bf414fab946a5.pdf
HYPER-GROWTH POTENTIAL

Website:
www.vycormedical.com
A few recent news: - they PR when they have something to PR
7/02/12
Vycor Medical, Inc. Announces Leadership Change, Appoints New Chief Executive Officer
1/17/2012
Vycor Medical's VBAS Granted Regulatory Clearance in China; Opening Order of $170,000 Shipped
You wanna make money right? Well time = money. So take the time to sit down, read this post. You will be impressed at the end at this legit company.
Non-penny penny stock with actual FDA approved, effective products
$100+ million dollar potential here
Company: Vycor Medical Inc.
Vycor Medical, Inc. is a medical device company committed to making neurological brain, spinal and other surgical procedures safer and more effective.
Vycor's innovative medical instruments are designed to optimize neurosurgical site access, reduce patient risk, accelerate recovery, and add tangible value to the professional medical community.
So basically, Vycor Medical was founded on this basic challenge: "There must be a better way". A better way to access surgical locations without unduly damaging surrounding tissue. A less invasive means to perform critical procedures, so that collateral trauma can be minimized and postoperative recovery accelerated. And a better breed of surgical access instrumentation for enhancing performance, safety and results such as the ViewSite Brain Access System, our premiere product line.
Operates in two complementary business units:
1. Vycor's ViewSite Brain Access System (VBAS), a next generation brain access system, is being used in brain surgeries in the US and internationally. The company is ISO 13485:2003 compliant, has U.S. FDA 510(k) clearance for brain and spine surgeries, CE Marking for the European Market and Canadian HPB licensing for brain, and has SFDA approval in China and most recently also regulatory approval in Japan.
The common instruments used in brain retraction procedures are ribbon or blade retractors (might I add VBAS costs less) that assist in the performance of some intraventricular and sub cortical procedures. Even though these instruments are still in use, neurosurgeons have a need for devices that provide better surgical outcomes – results that are beneficial to both the surgeon and the patient.
ViewSite™ Brain Access System is a revolutionary approach in brain retraction. Each ViewSite system consists of an introducer and a working channel port that allows the surgeon a seamless entry to the targeted site while distributing brain tissue evenly in a 360° dispersion pattern.
Other ViewSite benefits include superior binocular vision to see in and around the surgical site; multiple sizes in different widths and lengths to meet all surgical needs and compatibility with most surgical arms to avoid accidental displacement or movement during surgery.
--Cost-effective (shorter operating time, reduced post-operative recovery time)
--More efficient
--Less invasive (less damage to surrounding tissue)
--More accurate target access
--FDA approved
--Little to no competition
--Patents [#1 in Russia, #2 in China, 13 patents pending for its Brain and Spine technology and products in: the U.S. (3), Canada (2), Europe (2), India (2), Japan (2), and Hong Kong (2)]
VBAS vs. Standard Retractors
To give you a clearer idea on the effectiveness of VBAS, read below.
The goal of safely accessing the surgical site and creating a clear and stationary working channel to perform delicate procedures is what the ViewSite is all about. There is no pushing on the surgical site numerous times to see the selected area and no clutter of instrumentation during the procedure.
The ample working channel allows for multiple instruments to be used simultaneously while providing an excellent depth of view of the site as well as the brain wall. The working channel comes in four widths – 12mm, 17mm, 21mm and 28mm to meet your exact requirements.
Wanna see VBAS in action?
Video #1: http://vimeo.com/24741351
Video #2: http://vimeo.com/vycormedical/bulletcase
More info: VBAS Outlined by Dr. Quinones-Hinojosa, M.D.
How about Konstantin V. Slavin, MD?
Or Donald M. O'Rourke, MD, Ezriel E. Kornel, MD F.A.C.S, David J. Langer, MD, Ramin Rak, MD, Henry H. Woo, MD, Michael W. Weaver, MD, or John Ragheb, MD?
All have positive reviews of VBAS in the link below:
http://www.vycormedical.com/products/testimonials.html

There have been many successful surgeries performed with the ViewSite™ Brain Access System. Below is a listing* that includes these surgeries along with website information regarding clinical information on these surgeries:
Intracerebral Hematomas
www.nyp.org/health/cerebral-contusion-intracerebral-hematoma.html
Arterio-Venous Malformation (AVM)
www.ninds.nih.gov/disorders/avms/avms.htm
Cavernous Malformations
www.ninds.nih.gov/disorders/cavernous_malformation/cavernous_malformation.htm
Metastatic and Primary Brain Tumors (including frontal tumor resection)www.abta.org/siteFiles/SitePages/5E8399DBEEA8F53CBBBBF212C63AE113.pdf
Cerebral Cysts
www.abta.org/sitefiles/sitePages/4F77F57A14D6EBBE5C3FBC447B78FD57.pdf
Intraaxial Tumors and Lesions
www.ninds.nih.gov/find_people/groups/brain_tumor_prg/intraaxial.htm
Intraventricular Tumors
www.nyp.org/health/neuro_intraventricular.html
*It is important to note that the surgeries listed above are examples of applicable cases the ViewSite device can be used in. The ViewSite is not limited to these surgeries.
Not enough?
Well here are three clinical reports on VBAS.
Minimally Invasive Trans-Portal Resection ofDeep Intracranial Lesions by S. M. Raza, P. F. Recinos, J. Avendano, H. Adams, G. I. Jallo, and A. Quinones-Hinojosa
http://www.vycormedical.com/products/Journal%20of%20Minimally%20Invasive%20Neurosurgery%20Web.pdf
Vycor ViewSite TC®: Endoscope-guided Intraparenchimal Brain Tumor Resection
http://www.vycormedical.com/products/Prevedello%20case%201.pdf
Vycor ViewSite TC®: Endoscopic Intraparenchimal Brain Tumor Resection with Image Guidance
http://www.vycormedical.com/products/Dr%20Prevedello%20Case%202.pdf
So we’ve established that the product is top-of-the-line-amazing. Now, let’s talk about the distributors. The more the merrier. More distributors = more marketing = more revenue.
1. USA
2. Australia
3. China
4. Greece
5. Italy
6. Japan
7. Some of Germany
8. South Korea
9. Spain
10. Sweden
11. Switzerland
12. United Kingdom
13. Filed for registration in Russia
14. The Benelux (Belgium, Netherlands, and Luxembourg)
Any questions? Check the Frequently Asked Questions page:
http://www.vycormedical.com/products/faq.html
Not on there?
Vycor Medical Inc
6401 Congress Ave, Suite 140
Boca Raton, FL 33487
Phone: 561-558-2020
fax 631.794.2444
But wait, we’re not done. VBAS is not the online product Vycor produces. Their next, FDA-approved top-of-the-line-amazing product is:
2. NovaVision provides a home-based treatment of vision rehabilitation after stroke or brain injury called Vision Restoration Therapy (VRT™). TVRT is an FDA-cleared, patented, non-invasive medical device that enhances visual functions in patients with impairments due to stroke, surgical intervention, and traumatic brain injury (TBI). Some of the most common types of vision impairments that NovaVision VRT can treat include, Hemianopia, Scotoma, Quadrantanopia, and Amblyopia.
VRT is FDA 510(k) cleared and has CE Marking in Europe as a Class 1 device. It is the only FDA 501(k) cleared medical device in the U.S. aimed at the restoration of vision for neurologically induced vision loss.
While speech, physical, and occupational therapies are the long-standing treatment standards for stroke and TBI survivors, VRT is the first FDA-cleared clinical component of vision rehabilitation to physically enhance the visual field after a stroke or TBI.

Explanation video:
http://vimeo.com/39765566
The time between the injury and beginning therapy is not significant. There have been many successful outcomes for patients who suffered vision loss decades before receiving the therapy. One such case was that of a World War II veteran who benefited from VRT.
Soooooo how successful is VRT?
88% of VRT patients surveyed have mentioned at least one significant benefit as a result of performing VRT, such as improved reading, mobility, and in a few cases, driving again. A retrospective study of 161 patients from 16 medical centers across the US demonstrated that 76% of patients who completed this type of vision rehabilitation showed measurable improvements in their vision fields.
Other studies have produced similar results. As with all therapies individual results vary, and there is no way to predict how you will respond to VRT.
Clinical data have shown that the average vision gain from VRT vision rehabilitation is a five degree shift in the border of the blind field, which can have an exponential impact on a patient’s daily life. A 5 degree shift in the border of the blind field often leads to better reading, better mobility, less collisions, among other effects.
For the majority of patients, the improvement is not sufficient to return to driving, however, quite a few patients have achieved this goal. 88% of patients mention a significant improvement in quality of life.
Results do not appear to be age or gender dependent.
Now read some VRT Patient Testimonials:
http://www.novavision.com/images/stories/PDF/vrt-patient-grid-booklet.pdf
Dr. Michael Rosenberg, Neuro-Ophthalmologist administering VRT at New Jersey Neuroscience Institute at JFK Medical Center states that previously there were only ways to help stroke patients with vision loss adapt to their problem, and VRT actually addresses the problem.
VRT in action:
NovaVision Advisory Board:
1. Alvaro Pascual-Leone, MD, PhD, is Professor of Neurology at Harvard Medical School; Director of the Berenson-Allen Center for Noninvasive Brain Stimulation; Program Director of the Harvard-Thorndike Clinical Research Unit; and an Attending Neurologist at Beth Israel Deaconess Medical Center — all in Boston. He is a practicing behavioral neurologist and movement disorders specialist.
2. Jason J S Barton, MD PhD FRCPC - He is a neurologist, neuro-ophthalmologist and neuroscientist in visual cognition. He obtained his MD and his neurology residency at the University of British Columbia, and then did a clinical neuro-ophthalmology fellowship at the University of Iowa, followed by a PhD at the University of Toronto with Jim Sharpe.
3. Jose G. Romano, MD - Dr. Romano is an Associate Professor of Clinical Neurology, the Director of the Cerebrovascular Division at the University of Miami Miller School of Medicine and the Director of the Stroke Team at Jackson Memorial Hospital in Miami, Florida.
Acquisition of Sight Science, Ltd.
On January 4, 2012, NovaVision, entered into various agreements with Professor Arash Sahraie ("Prof. Sahraie") and the University of Aberdeen (Scotland) (the "University"), relating to the acquisition of all of the shares of Sight Science, Ltd. ("Sight Science") by NovaVision.
Sight Science was established in 2009 based on the research of Professor Arash Sahraie at the University of Aberdeen. Sight Science, which is owned jointly by Prof. Sahraie and the University of Aberdeen, provides an interactive computer-based therapy called Neuro-Eye Therapy ("NeET"), which patients administer at home.
To date, over 100 patients have utilized NeET. The Company has 5 patents granted in the UK, France, Germany, Switzerland and Singapore and 2 patents pending in the U.S. and Canada. Prof. Sahraie has conducted extensive research on blindsight and residual visual processing after brain injury, and is highly regarded in the field.
As part of the transaction, Prof. Sahraie agreed to join NovaVision as its Chief Scientific Officeron a part-time secondment basis from the University for a minimum of 5 years. Prof. Sahraie is responsible for driving NovaVision's scientific effort to develop and validate technologies in vision rehabilitation for visual field defects resulting from brain injury.
The acquisition also created a long-term relationship between the Company, NovaVision and the University, which is a leading medical research center. The University also entered into a Patent Agreement and Assignation Agreement with Sight Science where all of the intellectual property held by the University and which was related to Sight Science's business was assigned to Sight Science.
Both NovaVision's VRT and Sight Science's NeET work on the basis that repeated stimulation of the blind areas by either bright patches of light (VRT) or the specific spatial patterns (NeET) and can lead to increases in sensitivity of the blind areas. Patients progress after VRT appears to be initiated at the blind and sighted borders whereas NeET results in changes deep within the field damage. Both therapies are able to demonstrate improvements in both visual sensitivity and activities of daily living. The Company believes that these therapies are highly complementary.
--Niche market
--Little to no competition
--Proved (over 15 year of clinical research)
--Effective (in approximately 70% of cases patients using VRT system over a three to six month period experience significant recovery in at least one functional outcome -- the improvement conferred by VRT is permanent)
--Patents [ 23 granted patents are in the U.S. (11), Canada (2), Europe (4), Australia (1), China (2), Hong Kong (1), India (1) and Japan (1).
--The Company's 15 pending patents are in the U.S. and Canada (5), Europe (5), Australia (2), and Japan (3)]
--NovaVision substantial potential market: $2.6bn in US, over $20bn globally
--Reviewed and accepted by the scientific community
Revenue Growth:
More information:
Research report by Harbinger Research:
http://a.eqcdn.com/harbingerresearch/media/b767cf61ff1d00bcf70bf414fab946a5.pdf
HYPER-GROWTH POTENTIAL
Website:
www.vycormedical.com
A few recent news: - they PR when they have something to PR
7/02/12
Vycor Medical, Inc. Announces Leadership Change, Appoints New Chief Executive Officer
1/17/2012
Vycor Medical's VBAS Granted Regulatory Clearance in China; Opening Order of $170,000 Shipped
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.






