| Followers | 833 |
| Posts | 119895 |
| Boards Moderated | 17 |
| Alias Born | 09/05/2002 |
Monday, November 22, 2010 12:48:40 AM
Bayer/REGN Report ‘Positive’ Phase-3 VEGF-Trap-Eye Results in AMD
[While nominally successful at achieving statsig non-inferiority to Lucentis in both phase-3 trials on the primary endpoint of "maintained" vision (defined as a loss of <3 lines relative to baseline), I don’t think these results are enough to make VEGF-Trap-Eye a commercial success because only one of the three VEGF-Trap-Eye arms in only one of the two trials (i.e. 1 of 6 arms in toto) was statsig better than Lucentis on the secondary endpoint of mean lines of vision gained relative to baseline. Without clear-cut superiority to Lucentis, I find it hard to see how VEGF-Trap-Eye can effectively compete with off-label Avastin, which costs next to nothing and works about as well as Lucentis. CC today at 8:30am ET.]
http://finance.yahoo.com/news/Bayer-and-Regeneron-Report-prnews-657243616.html?x=0&.v=1
›In both studies, all regimens of VEGF Trap-Eye, including VEGF Trap-Eye dosed every two months, achieved primary endpoint compared to ranibizumab dosed every month
Regulatory applications for marketing approval planned in first-half of 2011
Monday November 22, 2010, 12:00 am
TARRYTOWN, N.Y. and BERLIN, Nov. 22, 2010 /PRNewswire-FirstCall/ -- Regeneron Pharmaceuticals, Inc. (Nasdaq:REGN) and Bayer HealthCare today announced that in two parallel Phase 3 studies in patients with the neovascular form of age-related macular degeneration (wet AMD), all regimens of VEGF Trap-Eye (aflibercept ophthalmic solution), including VEGF Trap-Eye dosed every two months, successfully met the primary endpoint compared to the current standard of care, ranibizumab dosed every month. The primary endpoint was statistical non-inferiority in the proportion of patients who maintained (or improved) vision over 52 weeks compared to ranibizumab.
Further results will be presented at the Angiogenesis Conference in February 2011. Bayer HealthCare and Regeneron are planning to submit regulatory applications for marketing approval in Europe and the U.S. in the first-half of 2011 based on the positive results of the VIEW 1 and VIEW 2 trials.
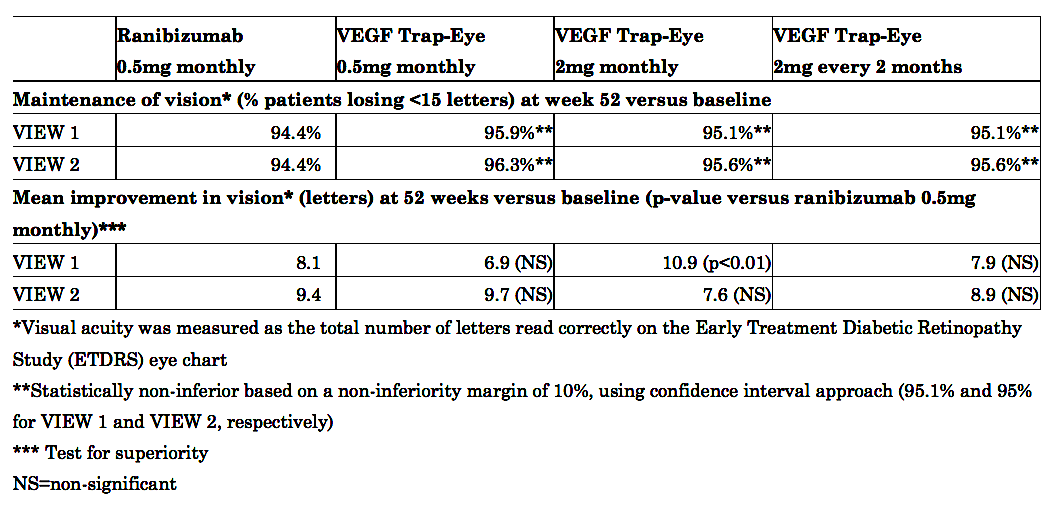
In the North American VIEW 1 study, 96 percent of patients receiving VEGF Trap-Eye 0.5mg monthly, 95 percent of patients receiving VEGF Trap-Eye 2mg monthly, and 95 percent of patients receiving VEGF Trap-Eye 2mg every two months achieved maintenance of vision compared to 94 percent of patients receiving ranibizumab 0.5mg dosed every month. In the international VIEW 2 study, 96 percent of patients receiving VEGF Trap-Eye 0.5mg monthly, 96 percent of patients receiving VEGF Trap-Eye 2mg monthly, and 96 percent of patients receiving VEGF Trap-Eye 2mg every two months achieved maintenance of vision compared to 94 percent of patients receiving ranibizumab 0.5mg dosed every month. Visual acuity was measured as a score based on the total number of letters read correctly on the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart, a standard chart used in research to measure visual acuity, over 52 weeks. Maintenance of vision was defined as losing fewer than three lines (equivalent to 15 letters) on the ETDRS eye chart.
"The currently available anti-VEGF therapies have significantly advanced the treatment of wet AMD, actually improving vision in many patients. However, monthly injections are required to optimize and maintain vision gain over the long-term," said Ursula Schmidt-Erfurth, M.D., Professor and Chair of the Department of Ophthalmology at the University Eye Hospital in Vienna, Austria and the VIEW 2 Principal Investigator. "The results of the VIEW studies indicate that VEGF Trap-Eye could establish a new treatment paradigm for the management of patients with wet AMD --- predictable every-other-month dosing without the need for intervening monitoring or dosing visits."
"In an effort to avoid the inconvenience of monthly office visits and the burden of monthly injections into the eye for their wet AMD patients, retinal specialists have tried to extend the benefits of the existing anti-VEGF therapy with less frequent dosing. A growing body of data suggests that this practice may result in inconsistent visual acuity outcomes," said Jeffrey Heier, M.D., a clinical ophthalmologist and retinal specialist at Ophthalmic Consultants of Boston, Assistant Professor of ophthalmology at Tufts School of Medicine, and Chair of the Steering Committee for the VIEW 1 trial. "A critical goal of these studies was to demonstrate that VEGF Trap-Eye could achieve robust improvements in vision and maintain them over time with a more convenient every-other-month dose. Achievement of this goal could be important for patients, care givers, and physicians."
In the VIEW 1 study, patients receiving VEGF Trap-Eye 2mg monthly achieved a statistically significant greater mean improvement in visual acuity at week 52 versus baseline (secondary endpoint), compared to ranibizumab 0.5mg monthly; patients receiving VEGF Trap-Eye 2mg monthly on average gained 10.9 letters, compared to a mean 8.1 letter gain with ranibizumab 0.5mg dosed every month (p<0.01). All other dose groups of VEGF Trap-Eye in the VIEW 1 study and all dose groups in the VIEW 2 study were not statistically different from ranibizumab in this secondary endpoint.
A generally favorable safety profile was observed for both VEGF Trap-Eye and ranibizumab. The incidence of ocular treatment emergent adverse events was balanced across all four treatment groups in both studies, with the most frequent events associated with the injection procedure, the underlying disease, and/or the aging process. The most frequent ocular adverse events were conjunctival hemorrhage, macular degeneration, eye pain, retinal hemorrhage, and vitreous floaters. The most frequent serious non-ocular adverse events were typical of those reported in this elderly population who receive intravitreal treatment for wet AMD; the most frequently reported events were falls, pneumonia, myocardial infarction, atrial fibrillation, breast cancer, and acute coronary syndrome. There were no notable differences among the study arms.
In the second year of the studies, patients in VIEW 1 and VIEW 2 will continue to be treated with the same dose per injection as in the first year but administered only every three months, or more often for any worsening of AMD, based on protocol-defined criteria (called "quarterly capped PRN" dosing).
About the VIEW Program
The VIEW (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD) program consists of two randomized, double-masked, Phase 3 clinical trials evaluating VEGF Trap-Eye in the treatment of the neovascular form of age-related macular degeneration (wet AMD). The VIEW 1 study, which randomized 1217 patients, is being conducted in the United States and Canada by Regeneron under a Special Protocol Assessment (SPA) with the U.S. Food and Drug Administration. The VIEW 2 study, which randomized 1240 patients, is being conducted in Europe, Asia Pacific, Japan, and Latin America by Bayer HealthCare. The study designs are essentially identical. The primary endpoint evaluation was conducted at 52 weeks.
In each of the studies, VEGF Trap-Eye was evaluated for its effect on maintaining and improving vision when dosed as an intravitreal injection on a schedule of 0.5mg monthly, 2mg monthly, or 2mg every two months (following three monthly loading doses), as compared with intravitreal ranibizumab administered 0.5mg every month during the first year of the studies. As-needed (PRN) dosing with both agents, with a dose administered at least every three months (but not more often than monthly), is being evaluated during the second year of each study. These studies are part of the global development program for VEGF Trap-Eye being conducted by Bayer HealthCare and Regeneron.
The primary endpoint of these non-inferiority studies is the proportion of patients treated with VEGF Trap-Eye who maintain visual acuity at the end of one year, compared to ranibizumab patients. Visual acuity is measured as a score based on the total number of letters read correctly on the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart, a standard chart used in research to measure visual acuity, over 52 weeks. Maintenance of vision is defined as losing fewer than three lines (equivalent to 15 letters) on the ETDRS chart.
The following table summarizes the VIEW 1 and VIEW 2 results for the primary and the first secondary endpoint pre-specified for testing:
About Wet AMD
Age-related Macular Degeneration (AMD) is a leading cause of acquired blindness. Macular degeneration is diagnosed as either dry (non-exudative) or wet (exudative). In wet AMD, new blood vessels grow beneath the retina and leak blood and fluid. This leakage causes disruption and dysfunction of the retina creating distortion and/or blind spots in central vision, and it can account for blindness in wet AMD patients. Wet AMD is the leading cause of blindness for people over the age of 65 in the U.S. and Europe.
About VEGF Trap-Eye
VEGF Trap-Eye is a fully human fusion protein, consisting of soluble VEGF receptors 1 and 2, that binds all forms of VEGF-A along with the related Placental Growth Factor (PlGF). VEGF Trap-Eye is a specific and highly potent blocker of these growth factors. VEGF Trap-Eye is specially purified and contains iso-osmotic buffer concentrations, allowing for injection into the eye.
VEGF Trap-Eye is also in Phase 3 development for the treatment of Central Retinal Vein Occlusion (CRVO), another major cause of blindness, in two identical studies. The COPERNICUS (COntrolled Phase 3 Evaluation of Repeated iNtravitreal administration of VEGF Trap-Eye In Central retinal vein occlusion: Utility and Safety) study is being led by Regeneron and the GALILEO (General Assessment Limiting InfiLtration of Exudates in central retinal vein Occlusion with VEGF Trap-Eye) study is being led by Bayer HealthCare. The primary endpoint of both studies is improvement in visual acuity versus baseline after six months of treatment. Initial data from the CRVO program are anticipated in early 2011.
VEGF Trap-Eye is also in Phase 2 development for the treatment of Diabetic Macular Edema (DME). In February 2010, Regeneron and Bayer HealthCare announced that treatment with VEGF Trap-Eye in the Phase 2 DA VINCI (DME And VEGF Trap-Eye: INvestigation of Clinical Impact) study demonstrated a statistically significant improvement in visual acuity versus baseline after six months of treatment compared to focal laser therapy, the primary endpoint of the study. Initial one-year results from this trial will be available before the end of this year.‹
[While nominally successful at achieving statsig non-inferiority to Lucentis in both phase-3 trials on the primary endpoint of "maintained" vision (defined as a loss of <3 lines relative to baseline), I don’t think these results are enough to make VEGF-Trap-Eye a commercial success because only one of the three VEGF-Trap-Eye arms in only one of the two trials (i.e. 1 of 6 arms in toto) was statsig better than Lucentis on the secondary endpoint of mean lines of vision gained relative to baseline. Without clear-cut superiority to Lucentis, I find it hard to see how VEGF-Trap-Eye can effectively compete with off-label Avastin, which costs next to nothing and works about as well as Lucentis. CC today at 8:30am ET.]
http://finance.yahoo.com/news/Bayer-and-Regeneron-Report-prnews-657243616.html?x=0&.v=1
›In both studies, all regimens of VEGF Trap-Eye, including VEGF Trap-Eye dosed every two months, achieved primary endpoint compared to ranibizumab dosed every month
Regulatory applications for marketing approval planned in first-half of 2011
Monday November 22, 2010, 12:00 am
TARRYTOWN, N.Y. and BERLIN, Nov. 22, 2010 /PRNewswire-FirstCall/ -- Regeneron Pharmaceuticals, Inc. (Nasdaq:REGN) and Bayer HealthCare today announced that in two parallel Phase 3 studies in patients with the neovascular form of age-related macular degeneration (wet AMD), all regimens of VEGF Trap-Eye (aflibercept ophthalmic solution), including VEGF Trap-Eye dosed every two months, successfully met the primary endpoint compared to the current standard of care, ranibizumab dosed every month. The primary endpoint was statistical non-inferiority in the proportion of patients who maintained (or improved) vision over 52 weeks compared to ranibizumab.
Further results will be presented at the Angiogenesis Conference in February 2011. Bayer HealthCare and Regeneron are planning to submit regulatory applications for marketing approval in Europe and the U.S. in the first-half of 2011 based on the positive results of the VIEW 1 and VIEW 2 trials.
In the North American VIEW 1 study, 96 percent of patients receiving VEGF Trap-Eye 0.5mg monthly, 95 percent of patients receiving VEGF Trap-Eye 2mg monthly, and 95 percent of patients receiving VEGF Trap-Eye 2mg every two months achieved maintenance of vision compared to 94 percent of patients receiving ranibizumab 0.5mg dosed every month. In the international VIEW 2 study, 96 percent of patients receiving VEGF Trap-Eye 0.5mg monthly, 96 percent of patients receiving VEGF Trap-Eye 2mg monthly, and 96 percent of patients receiving VEGF Trap-Eye 2mg every two months achieved maintenance of vision compared to 94 percent of patients receiving ranibizumab 0.5mg dosed every month. Visual acuity was measured as a score based on the total number of letters read correctly on the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart, a standard chart used in research to measure visual acuity, over 52 weeks. Maintenance of vision was defined as losing fewer than three lines (equivalent to 15 letters) on the ETDRS eye chart.
"The currently available anti-VEGF therapies have significantly advanced the treatment of wet AMD, actually improving vision in many patients. However, monthly injections are required to optimize and maintain vision gain over the long-term," said Ursula Schmidt-Erfurth, M.D., Professor and Chair of the Department of Ophthalmology at the University Eye Hospital in Vienna, Austria and the VIEW 2 Principal Investigator. "The results of the VIEW studies indicate that VEGF Trap-Eye could establish a new treatment paradigm for the management of patients with wet AMD --- predictable every-other-month dosing without the need for intervening monitoring or dosing visits."
"In an effort to avoid the inconvenience of monthly office visits and the burden of monthly injections into the eye for their wet AMD patients, retinal specialists have tried to extend the benefits of the existing anti-VEGF therapy with less frequent dosing. A growing body of data suggests that this practice may result in inconsistent visual acuity outcomes," said Jeffrey Heier, M.D., a clinical ophthalmologist and retinal specialist at Ophthalmic Consultants of Boston, Assistant Professor of ophthalmology at Tufts School of Medicine, and Chair of the Steering Committee for the VIEW 1 trial. "A critical goal of these studies was to demonstrate that VEGF Trap-Eye could achieve robust improvements in vision and maintain them over time with a more convenient every-other-month dose. Achievement of this goal could be important for patients, care givers, and physicians."
In the VIEW 1 study, patients receiving VEGF Trap-Eye 2mg monthly achieved a statistically significant greater mean improvement in visual acuity at week 52 versus baseline (secondary endpoint), compared to ranibizumab 0.5mg monthly; patients receiving VEGF Trap-Eye 2mg monthly on average gained 10.9 letters, compared to a mean 8.1 letter gain with ranibizumab 0.5mg dosed every month (p<0.01). All other dose groups of VEGF Trap-Eye in the VIEW 1 study and all dose groups in the VIEW 2 study were not statistically different from ranibizumab in this secondary endpoint.
A generally favorable safety profile was observed for both VEGF Trap-Eye and ranibizumab. The incidence of ocular treatment emergent adverse events was balanced across all four treatment groups in both studies, with the most frequent events associated with the injection procedure, the underlying disease, and/or the aging process. The most frequent ocular adverse events were conjunctival hemorrhage, macular degeneration, eye pain, retinal hemorrhage, and vitreous floaters. The most frequent serious non-ocular adverse events were typical of those reported in this elderly population who receive intravitreal treatment for wet AMD; the most frequently reported events were falls, pneumonia, myocardial infarction, atrial fibrillation, breast cancer, and acute coronary syndrome. There were no notable differences among the study arms.
In the second year of the studies, patients in VIEW 1 and VIEW 2 will continue to be treated with the same dose per injection as in the first year but administered only every three months, or more often for any worsening of AMD, based on protocol-defined criteria (called "quarterly capped PRN" dosing).
About the VIEW Program
The VIEW (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD) program consists of two randomized, double-masked, Phase 3 clinical trials evaluating VEGF Trap-Eye in the treatment of the neovascular form of age-related macular degeneration (wet AMD). The VIEW 1 study, which randomized 1217 patients, is being conducted in the United States and Canada by Regeneron under a Special Protocol Assessment (SPA) with the U.S. Food and Drug Administration. The VIEW 2 study, which randomized 1240 patients, is being conducted in Europe, Asia Pacific, Japan, and Latin America by Bayer HealthCare. The study designs are essentially identical. The primary endpoint evaluation was conducted at 52 weeks.
In each of the studies, VEGF Trap-Eye was evaluated for its effect on maintaining and improving vision when dosed as an intravitreal injection on a schedule of 0.5mg monthly, 2mg monthly, or 2mg every two months (following three monthly loading doses), as compared with intravitreal ranibizumab administered 0.5mg every month during the first year of the studies. As-needed (PRN) dosing with both agents, with a dose administered at least every three months (but not more often than monthly), is being evaluated during the second year of each study. These studies are part of the global development program for VEGF Trap-Eye being conducted by Bayer HealthCare and Regeneron.
The primary endpoint of these non-inferiority studies is the proportion of patients treated with VEGF Trap-Eye who maintain visual acuity at the end of one year, compared to ranibizumab patients. Visual acuity is measured as a score based on the total number of letters read correctly on the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart, a standard chart used in research to measure visual acuity, over 52 weeks. Maintenance of vision is defined as losing fewer than three lines (equivalent to 15 letters) on the ETDRS chart.
The following table summarizes the VIEW 1 and VIEW 2 results for the primary and the first secondary endpoint pre-specified for testing:

About Wet AMD
Age-related Macular Degeneration (AMD) is a leading cause of acquired blindness. Macular degeneration is diagnosed as either dry (non-exudative) or wet (exudative). In wet AMD, new blood vessels grow beneath the retina and leak blood and fluid. This leakage causes disruption and dysfunction of the retina creating distortion and/or blind spots in central vision, and it can account for blindness in wet AMD patients. Wet AMD is the leading cause of blindness for people over the age of 65 in the U.S. and Europe.
About VEGF Trap-Eye
VEGF Trap-Eye is a fully human fusion protein, consisting of soluble VEGF receptors 1 and 2, that binds all forms of VEGF-A along with the related Placental Growth Factor (PlGF). VEGF Trap-Eye is a specific and highly potent blocker of these growth factors. VEGF Trap-Eye is specially purified and contains iso-osmotic buffer concentrations, allowing for injection into the eye.
VEGF Trap-Eye is also in Phase 3 development for the treatment of Central Retinal Vein Occlusion (CRVO), another major cause of blindness, in two identical studies. The COPERNICUS (COntrolled Phase 3 Evaluation of Repeated iNtravitreal administration of VEGF Trap-Eye In Central retinal vein occlusion: Utility and Safety) study is being led by Regeneron and the GALILEO (General Assessment Limiting InfiLtration of Exudates in central retinal vein Occlusion with VEGF Trap-Eye) study is being led by Bayer HealthCare. The primary endpoint of both studies is improvement in visual acuity versus baseline after six months of treatment. Initial data from the CRVO program are anticipated in early 2011.
VEGF Trap-Eye is also in Phase 2 development for the treatment of Diabetic Macular Edema (DME). In February 2010, Regeneron and Bayer HealthCare announced that treatment with VEGF Trap-Eye in the Phase 2 DA VINCI (DME And VEGF Trap-Eye: INvestigation of Clinical Impact) study demonstrated a statistically significant improvement in visual acuity versus baseline after six months of treatment compared to focal laser therapy, the primary endpoint of the study. Initial one-year results from this trial will be available before the end of this year.‹
“The efficient-market hypothesis may be
the foremost piece of B.S. ever promulgated
in any area of human knowledge!”
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.










