Monday, March 25, 2024 10:40:41 AM
United Health Products' Press Releases Cannot Be Taken At Face Value
Oct. 30, 2019 10:00 AM ETUnited Health Products, Inc. (UEEC)9 Comments
White Diamond Research profile picture
White Diamond Research
2.52K Followers
Summary
United Health Products releases many positive PRs with claims that never happen and doesn’t report negative news.
We found many of UEEC's reported distribution deals didn't materialize, and one distribution company doesn't even appear to exist.
We failed to find any UEEC FDA Class II or Class III approvals, despite the company's investor relations saying otherwise.
UEEC’s October 18th announcement of positive clinical results omitted critical safety outcomes, suggesting its PMA marketing application will be denied.
On December 31, 2019, the CEO will be granted 15% of the outstanding shares for no performance reason.
United Health Products (OTCPK:UEEC) is a $200M market cap company trading on the pink sheets, with only one product, its HemoStyp gauze. HemoStyp is an ordinary wound gauze, manufactured in China, and a commercial flop. Sales are miniscule, with $46K in revenues for 2018 and $25K in revenues through the first half of 2019. We published an article on 8/27/19 telling about the history of the company and HemoStyp, and how absurd the company’s valuation is.
In this follow-up report, we took a look at past press releases ("PRs") from the company claiming they have business dealings and contracts with various distributors and medical device vendors. Many of these business dealings appear to have never come to fruition, and even at least one of the companies doesn’t appear to exist. Looking at the past seven years of the company’s PR history, we have not seen a single negative or even neutral statement updating shareholders on its endeavors. This is despite clearly having so many distribution partnership and FDA setbacks. We show our research on UEEC’s PRs in this report.
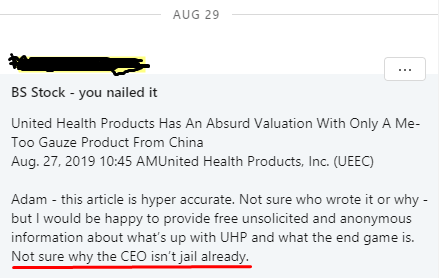
We also have some outside validation that we are on the right track with our findings on UEEC. Someone who used to work with the company, who requested anonymity, contacted us on LinkedIn after we published our previous article and stated (emphasis ours):
Source: LinkedIn Message
A statement that strongly motivated us to do a deeper dive into UEEC.
A Deep Dive Into United Health Products Shows Many Questionable Press Releases
A look into UEEC's PRs shows that many are distribution deals that didn't materialize and FDA submissions with no follow-up news.
The source that we quoted earlier had some really negative, and shocking, things to say about UEEC and its CEO, Douglas Beplate. Here’s what he told us over the phone:
This is my industry. United Health Products was a mistake to work with, but it’s part of what made me successful, I learn from my mistakes.
This is a BS company. There’s no truth to it, the product’s shady. Doug Beplate is a master fibber. He puts out PRs to pump his stock up. Nothing is going to happen, they aren’t doing anything. This is all a shell game. The $25K in quarterly revenues could be a money shuffle. I’ve showed it to doctors myself, nobody uses it
It’s easy for me to sit in a basement in a bunker in Utah, see what other companies who are successful put out in PRs, and just do the same thing for my company. That takes zero capital. And that’s what he does, he puts in whatever sounds good in the press. There’s a lot of hoopla with PRs, but as far as real revenue on the books, there’s nothing there. Other companies have medical journals, links to the data, etc., but there’s nothing for United Health Products.
They are never going to get Class III approval. That is complete BS. Because you have to spend the money to do the research to get the Class III approval. Class III is placing it into the body cavity. And everyone I’ve talked to said “there’s no way”. You can put it into a body cavity, like a deep wound, and sew it up. But you can’t do that with this product. It’s not indicated for that, it doesn’t have an indication for anything, it’s a wound gauze, that’s it, it’s a gel infused mesh. I’ll be honest with you, the docs at San Antonio told me, that based on the advertised blood stopping ability of HemoStyp, you can get the same results from taking a T-shirt and putting it on the wound and holding pressure to it. There’s nothing special about this product.
Of course, the above statement is just one person’s point of view about UEEC and its CEO who had a bad experience working with the company. But our research has shown us that, for the most part, what this anonymous source has told us is true. Here are some questions that arose from our research that we would like to ask United Health Products:
On 1/21/16 and 5/16/16, UEEC issued PRs stating that it made a distribution agreement with a company named Optimal Government Resources/Services to sell HemoStyp to the US government. Our research shows that “Optimal Government Resources/Services” does not appear to exist (evidence shown in the next section). Can UEEC show any evidence that this company exists or ever did? Also, we did a simple Google search and found that the DAPA contract number UEEC says is theirs actually belongs to another medical product company, TrillaMed (evidence shown in the next section). How does UEEC explain this?
2. UEEC reported on 10/10/17 that its “Expanded Indication Submission Class II application continues to progress” for HemoStyp. In this same PR, UEEC reported,
the company is pleased to announce that it has applied to the FDA’s new and innovative CtQ Pilot Program premarket approval "PMA" (Class III internal usage) for HemoStyp.
This PR was over two years ago, and there hasn’t been any follow-up on the results of the applications. How did the FDA respond to either application? Were they rejected? We submitted a request to the FDA and will update when we hear back.
3. UEEC reported on 12/12/17 that it’s:
applying to have HemoStyp designated as a Class III medical device with Australia’s Therapeutics Goods Administration ("TGA", counterpart to the US FDA).
The PR further stated:
The company’s Australian TGA consultants believe that UHP has the requisite test data and documentation to obtain rapid approval in Australia, and that HemoStyp could obtain approval for use in the Australian market within 30 days.
This Australia TGA application was also mentioned in the 2017 10-K. There has been no follow-up to this PR. It has been almost two years, despite the PR saying approval could happen within 30 days. Did the TGA respond to or reject this application? Why did the consultants believe UEEC had enough data for approval? There is no mention of any revenues in Australia in UEEC’s quarterly or annual reports.
4. What happened to the 5-year contract with Total Resources International ("TRI"), where UEEC stated in a PR on 3/17/15 that sales generated will be a minimum of $3M in year one and expected to gross over $20M in during the life of the contract?
UEEC reported sales were $53.3K in 2015, $242K in 2016, $646K in 2017, $46K in 2018 and so far $25K in the first half of 2019. Nowhere near the “expected $20M” over five years.
5. What happened to HemoStyp sales on henryschein.com and the Australian Henry Schein catalogue? UEEC claimed to have signed an agreement with Henry Schein on 8/24/15 here, and Henry Schein Halas on 5/2/16 here to sell HemoStyp. HemoStyp isn’t listed on either online catalogue. We called up Henry Schein, and they said they have never had a contract with UEEC to sell HemoStyp.
6. On 7/9/18, UEEC issued a PR stating:
UEEC today announced that it has been accepted as a Walmart.com and Jet.com supplier, and will offer three HemoStyp wound care products for online retail sale.
Doing a search on Walmart.com and Jet.com, we found that HemoStyp isn’t sold on either website. Why is this? Was the company’s supplier status revoked? We emailed Walmart.com (WMT), and the company replied that indeed they do not sell HemoStyp.
7. On 10/25/18, UEEC announced it hired Societe Generale (OTCPK:SCGLF) to advise the company on strategic alternatives. It states:
In connection with the FDA PMA Class III approval process for HemoStyp, UHP has been contacted by several medical technology companies that are active in the surgical equipment and hemostatic products sectors, and who have expressed an interest in the Company's products and business strategy.
This PR was over one year ago now. UEEC is not a complicated company with only one simple gauze product, so it shouldn’t take this long. UEEC still mentions the possibility of an acquisition or strategic partnership in PRs.
In a recent PR on 10/17/19, it states:
in anticipation of a successful FDA Class III PMA application approval, the potential acquisition of the Company or a strategic partnership.
In a PR released the next day, on 10/18/19, it again states:
UHP is continuing its discussions with well positioned candidates interested in acquisition of the Company or partnerships
We would like UEEC to show evidence that this is a true statement. Which medical technology companies have shown interest? Why has it taken over a year of discussions and still there have been no updates on a collaboration or merger? What exactly is the company still “discussing” with suitors after over a year of discussions?
Why would UEEC be acquired at this current valuation given that present HemoStyp sales are miniscule and the probability of FDA approval is low? We think any acquisition talks the company is in aren't serious and there won't be any merger or acquisition.
8. On 4/7/15, UEEC issued a PR stating:
United Health Products, Inc. (OTCPK:UEEC) has received the final laboratory test results and pathology reports for recent porcine testing.
Douglas Beplate, CEO said, "The results are remarkable in that they not only show that HemoStyp® is effective at rapid hemostasis in a wound site, but prove it is totally absorbable into the body." He added, "In my opinion HemoStyp® promotes vascular genesis and healing.”
The tests reports were furnished to the U.S. Military will be posted for viewing on the United Health Products Inc. website.”
But the study was never posted on UEEC’s website. If this swine study was favorable and submitted to the US Military, it should have been published. There would be no reason to hide favorable results from the study which UEEC supposedly had done. Why didn’t UEEC publish the results like the PR said it would?
These 8 questions are just some that we have. Further digging would certainly lead to more.
More Details On The Press Release Questions
In this section, we go into more detail on two of our questions, questions #1 and #2. If needed, look back at the previous section for a summary of each question.
Question #1
For question #1, we did more digging on what we believe to be a fictional company, Optimal Government Services/Resources. In UEEC’s PR on 5/16/16, it states:
Optimal Government Resources has signed their distribution agreement and placed an opening stocking order. In conjunction with this agreement, UHP HemoStyp® products are now listed under the DAPA contract #SP0200-09-H-0037.
Doing a simple Google search for this DAPA (Distribution and Pricing Agreement) contract number, we found it belongs to a contract by another medical product company, TrillaMed. This page shows a newsletter from TrillaMed, and at the bottom, it shows:
Source: TrillaMed Newsletter
We underlined the exact same DAPA contract number in UEEC’s PR, but it belongs to TrillaMed.
Our research showed us that each DAPA number is unique to each company specifically. It’s not unique to a larger contract that multiple companies can pile on, because every company has their specific number. Therefore, either TrillaMed has the number wrong, or UEEC has it wrong. There is no news anywhere, from UEEC or TrillaMed, that these companies have ever worked together or that TrillaMed was distributing HemoStyp for UEEC. We have contacted TrillaMed and will update when we hear back.
Doing a Google search for both Optimal Government Services and Optimal Government Resources, we found no evidence anywhere that there exists a company by either name. This webpage shows a company with a similar name that does government contracts for medical products. We called up the company and they said the name of their company isn't "Optimal Government Services/Resources", but just "Optimal" or "Golden Max". And of course, the company has a website, which UEEC didn't include in their PR.
To do business with the Federal Government, a company must have an active registration with the System for Award Management (SAM). We searched for Optimal Government Services and Optimal Government Resources on sam.gov, and neither one has a registration there. Screenshot shown below:
Source: sam.gov
Question #2
In regards to question #2 about the 10/10/17 PR on the FDA marketing applications, UEEC had some follow-up PRs.
On 12/4/17, UEEC issued a PR stating:
it is proceeding with its application for HemoStyp under the FDA’s new and innovative CtQ Pilot Program. The FDA selected UHP’s HemoStyp as only one of nine participants for the program.
Concurrent with its CtQ Pilot Program participation, UHP reaffirms that its current Class II application – Expanded Indication submission with the US FDA-- continues to progress, and is in an advanced stage of review
On 2/5/18, UEEC issued a PR stating:
United Health Products, Inc. (UHP) (OTC:OTCPK:UEEC) today announced that, following its January 17 face-to- face meeting with FDA experts and officials, it has completed and submitted all materials relevant for the premarket approval (PMA) for HemoStyp. UHP has submitted additional inspection and registration forms to the CtQ Pilot program for final approval and confirmation. The PMA submission is for the approval of Class III indication and internal surgical use of Hemostyp.
The latter PR was over a year and a half ago, and UEEC has still not announced PMA approval for HemoStyp. Why have there not been any updates to this FDA meeting?
United Health Products Investor Relations Rep Gave Us More False Info
To figure out what’s going on with UEEC and these questionable PRs, we called up the company’s investor relations rep. Unfortunately, he didn’t appear to play it straight with us and gave more false info.
First, we tried calling the company, and it goes to a voicemail that doesn’t even mention the company's name. We left a message anyways to try and speak with Doug Beplate to try and find the answers. Then, we called the investor relations firm, Pan Consultants, and spoke with Phillipe Niemitz.
We believe multiple comments by Niemitz, specifically two comments regarding FDA approval for HemoStyp, are not true. We checked the 510K database on the FDA website here. We did a search for “HemoStyp”, “United Health”, and “UHP”, and we didn’t find any device approval on those names. Looking at its press release history, UEEC has never released a PR claiming that they have received FDA approval for any indication.
See the full IR interview transcript here.
HemoStyp Is A Commercial Flop
As everyone who has read UEEC’s financial statements knows, its HemoStyp gauze is a commercial flop. The company has only generated $46K in revenue in 2018. In the first two quarters of 2019, it has only generated $25K in revenue so far. Despite poor commercial performance, the CEO is on track to receive a stock bonus that will be worth in the $10s of million if the Company manages to keep the stock price up till the end of the year. This CEO bonus is stated in the company's latest 10-K.
United Health Product’s PRs Describing Its HemoStyp PMA Application Process Are Bizarre, Suggesting A Denial Is Likely
UEEC’s announcement of the PMA submission was released on 10/4/19 stating:
UEEC today announced that it has submitted a Premarket Approval (PMA) application for Class III approval to the FDA for HemoStyp.
This was seemingly very significant news about an important milestone concluding clinical study data analysis and submission. The text of the announcement was, however, very generic and reserved. It further states:
The PMA program confirms the safety and efficacy of a product. If approved, UHP expects that HemoStyp will be authorized for use in surgical procedures in abdominal, cardiovascular, thoracic and vascular surgeries per UHP's PMA filed Instructions For Use.
This October 4th PR mentions nothing about the results of the clinical studies, simply noting that the FDA submission has occurred.
A pre-market application (PMA) application is a very detailed and stringent process. As described in drugwatch.com, the PMA includes:
Source: drugwatch.com
Therefore, the October 4th PMA submission should have included clinical data, including a statistical analysis of its results.
Then, on 10/18/19, a relevant, yet bizarre, PR was announced, claiming positive results of the HemoStyp clinical study. We are puzzled because this announcement comes two weeks after the PMA submission on 10/4/19.
The PR says the final analysis report has been received, and it is from an independent reviewer (does “independent” mean that UEEC didn't pay for this analysis?). It further states:
In summary the independent statistical reviewer (website here) stated:
“For the primary analysis comparing HemoStyp versus Surgicel for hemostasis in 10 minutes, both non-inferiority and superiority were satisfied in both ITT population and PP population. For the secondary analysis, HemoStyp was significantly better than Surgicel with respect to the percentage achieving hemostasis at 2 minutes, 5 minutes, and 10 minutes.”
It further states:
UEEC... today announced that it has received the final report from an independent review of the results of its human clinical trial.
The way the above sentence is worded, it seems as though UEEC had just received the statistical results on October 18 or a day before. It doesn't say that this report was received and sent to the FDA 2 weeks earlier on October 4th. But if it was in fact sent to the FDA in tandem with the October 4th submission, then it is strangely communicated.
Why would they do the analysis after they already sent the application? There is such a thing as a PMA Amendment if the applicant is revising existing information or providing additional information. But UEEC didn’t say that they were doing a PMA Amendment. It is sometimes required to continue statistical analysis after the PMA submission if the clinical study is continuing and the longer patient follow-up is recorded. But according to the UEEC protocol, the follow-up was 30 days and that was finished long ago.
Because this “independent” statistical reviewer apparently gave its analysis after UEEC already submitted its application to the FDA, we would take it with a grain of salt. If it were a relevant review, it should have been included in the PMA application on October 4th. If this analysis was not included, we also wonder then what analysis was included and why the results of that analysis were not mentioned in the PR from October 4th.
But what is the most alarming to us in the 10/18/19 announcement is that the PR went silent on the very important product safety outcomes. From the UEEC clinical protocol, described on its clinicaltrials.gov page we learned about four secondary study outcomes:
Source: clinicaltrials.gov
While the Primary outcome and the Secondary outcome #1 in the UEEC study protocol are efficacy related, the Secondary #2, #3, and #4 outcomes are all safety outcomes and are of critical importance for FDA approval. The October 18th PR only addresses success of the Primary outcome and Secondary outcome #1. The PR didn't say if the study has shown a non-inferiority or superiority of HemoStyp vs Surgicel in the secondary outcomes #2-4. The history of UEEC only disclosing positive news makes us believe that the omission of results relevant to outcomes #2-4 could have been on purpose, because the clinical study likely showed the inferior performance of HemoStyp vs Surgicel in these outcome measures.
The statistician was very specific to claim non-inferiority and superiority of HemoStyp only in the initial hemostasis after application of HemoStyp to the wound. It is silent on what has happened after the initial hemostasis was achieved.
HemoStyp, according to UEEC, quickly dissolves in an aqueous environment due to its unique degradation properties. We can find this on the company’s website.
From UEEC’s product testing page:
The data obtained during analysis of sample UHP Box of 2” x 2” Pouches indicates the material begins to dissolve in water within one minute. The sample is completely dissolved within 24 hours. Not enough material remains for analysis at the 24 hour time point.
If HemoStyp dissolves too soon, the surgical wound would start bleeding again, and this would be a very serious safety issue.
Imagine a major disaster when bleeding re-occurs after the surgeon has already closed the outmost patient skin layer! The outcomes 2-4 directly address this key safety concern, describing in quantitative terms how well the initially achieved hemostasis is maintained during the surgery. Outcome 4 is about the failure of initial hemostasis, as measured in reoperations, during the one month after surgery. It seems to us that a hemostatic agent that is quick to achieve initial hemostasis, but rapidly dissolves and may frequently fail later resulting in recurrence of bleeding, is a major health hazard. Why would the FDA approve HemoStyp, if it indeed fails to maintain hemostasis, knowing that Surgicel is already a reasonably effective and safe FDA approved product?
From all these observations, we conclude that the clinical study of HemoStyp versus Surgicel was likely a failure. However, if and when its PMA application gets rejected, we doubt that shareholders will hear about it. We expect it will be just like when UEEC applied for PMA approval for Class III in late 2017, there will just not be any updates, like it never happened.
On New Year's Eve, The CEO Will Be Granted 15% Of The Outstanding Shares For No Performance Reason
In the latest 10-Q, it states:
The Company, by board resolution, approved an executive compensation stock bonus package for Mr. Beplate such that upon the sale of all or substantially all of the assets of the Company or other change in control or merger transaction in which the Company is involved, or in the event that no such transaction occurs by December 31, 2019, Mr. Beplate shall receive an amount equal to 15% post issuance of the then outstanding shares of the Company's common stock on a fully diluted basis.
In a nutshell, what the above passage says is if the company gets acquired, or it doesn’t get acquired, UEEC’s CEO, Douglas Beplate, will be awarded an amount of shares equal to 15% of the outstanding shares of UEEC.
So, whether Beplate performs well and gets the company acquired, or performs poorly, he still gets gifted all those shares. Which if the company has a $200M market cap, the shares are worth about $30M. We don’t believe he did anything to deserve this kind of a bonus. And to make matters worse for shareholders, there isn’t any kind of lockup period mentioned. Therefore, Beplate can immediately sell as many shares as he wants in the open market. This will be an egregious New Year's Eve gift to the CEO at shareholders' expense.
We believe shareholders should be very worried about this transaction. The company hasn’t accomplished anything to this date. It’s generating revenues of about $50K per year. Yet, Beplate is receiving a bonus this year that a CEO of a blue chip company would be envious of.
Conclusion
In this report, we began by quoting a disturbing interview with a source who used to work for United Health Products. He claimed the CEO Beplate is a “master fibber”, and that there is nothing going on with UEEC, it’s just a “shell game”. We included many PRs the company put out over the past few years stating that they had distribution agreements and marketing applications that didn’t appear to go anywhere, including PMA submissions for HemoStyp Class III approval. But the company never issued a PR claiming a distribution agreement fell through or a marketing application was denied. It was like they never happened, and the company just put out additional PRs that again turned out to be nothing. What does the company have to show today for all these PRs? A miniscule $50K of revenues per year. To add insult to injury to shareholders, the board agreed to reward Beplate for all these failures with a bonus of 15% of the outstanding shares.
Right now, shareholders are putting all their hopes on PMA approval happening for a Class III indication. We believe the PMA application likely be denied by the FDA, and since the company’s current sales are almost zero, that would make the company worth close to zero.
We included our observations in this report on why we believe the PMA application will get denied. There was a bizarre PR of an independent analysis of the study that was released weeks after the PMA application was submitted to the FDA, when an analysis should’ve been included in the application. Neither the complete study results nor a complete analysis have been posted anywhere. Furthermore, three of the four secondary outcomes, related to product safety, were omitted in the quote. From the nature of HemoStyp, we speculate that it may dissolve before the internal wound has healed, which could result in a renewed bleeding. If this is true about the HemoStyp product, we think the FDA will deny PMA approval.
Recent UEEC News
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 05/09/2024 08:16:02 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 11/13/2023 10:05:42 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 08/09/2023 09:10:10 PM
Glidelogic Corp. Becomes TikTok Shop Partner, Opening a New Chapter in E-commerce Services • GDLG • Jul 5, 2024 7:09 AM
Freedom Holdings Corporate Update; Announces Management Has Signed Letter of Intent • FHLD • Jul 3, 2024 9:00 AM
EWRC's 21 Moves Gaming Studios Moves to SONY Pictures Studios and Green Lights Development of a Third Upcoming Game • EWRC • Jul 2, 2024 8:00 AM
BNCM and DELEX Healthcare Group Announce Strategic Merger to Drive Expansion and Growth • BNCM • Jul 2, 2024 7:19 AM
NUBURU Announces Upcoming TV Interview Featuring CEO Brian Knaley on Fox Business, Bloomberg TV, and Newsmax TV as Sponsored Programming • BURU • Jul 1, 2024 1:57 PM
Mass Megawatts Announces $220,500 Debt Cancellation Agreement to Improve Financing and Sales of a New Product to be Announced on July 11 • MMMW • Jun 28, 2024 7:30 AM









