Wednesday, January 27, 2021 1:09:26 AM
Alongside revenue generated from consults, this acquisition provides MGC Pharma with an operating platform with both import and export capacity that will significantly expand market access and provide control of the supply chain from manufacturing through to patients.
The acquisition also allows the Company to continue providing its high-quality GMP certified medications to patients in Australia and further improves profit margins while keeping product at the current competitive prices.
The acquisition of the MCC Assets is the next step in building on-the-ground distribution assets allowing the Company to wholesale and distribute directly to other clinics and pharmacies to reduce storage and distribution costs.
Launch of CannEpil® App
As part of its ongoing work with the Royal Melbourne Institute of Technology (RMIT), MGC Pharma launched the CannEpil® App, and is providing medical access to the International Library of Cannabinoids (ILC).
The App is a cross platform application available to download from both the Apple App Store and Google Play Store and is designed to be used by patients (or the patient’s guardian) taking CannEpil® as a prescription treatment. The App will record patient responses to medical questionnaires as part of their treatment plan and the treating practitioner will be able to view the responses in real-time.
The ILC is a world first centralised platform compiling the diverse range of existing data on the therapeutic benefits of cannabinoids. The ILC database has been designed to collect comprehensive information about clinical trials, including details of diseases and follow up treatments, as well as product identifiers, including genetics, grow conditions and chemical profile to provide doctors with an encyclopaedia of exhaustive information on the best treatment for patients using cannabinoids.
First shipment of MP Line products directly to patients in Brazil
MGC Pharma’s first batch of MP Line products were shipped directly to patients in Brazil in October 2020, through its binding supply and distribution agreement with Brazil-based ONIX Empreendimentos e Participações (‘ONIX’). MGC Pharma is the first company globally to ship high THC formulations directly to a patient’s door in Brazil, without the need to visit a pharmacy.
The shipment was completed under Brazil’s Compassionate Use Program following the receipt of patients’ prescriptions provided by an ONIX referring doctor. ONIX currently has more than 100 referring doctors in Brazil able to prescribe cannabinoid products under the Compassionate Use Program and is targeting to have over 1,000 referring doctors by mid-2021.
Research and Development
Completion of Phase II clinical trial on COVID-19 patients
MGC Pharma’s Phase II double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of anti-inflammatory treatment, ArtemiCTM, on 50 patients diagnosed with COVID-19 has completed.
The trial included 50 patients of which 33 were in the treatment group and 17 in the placebo group and took place across three independent hospital sites across Israel and India.
The full results have demonstrated to improve the health status of COVID-19 patients delivering a NEWS score of less than or equal to 2. None of the patients in the treatment group required additional oxygen, mechanical ventilation or admission to intensive care where all of these events were reported in the placebo group. The average NEWS score of patients in the placebo group was 2.25 statistically significantly higher (p<0.04) than in the treatment group – 0/5.
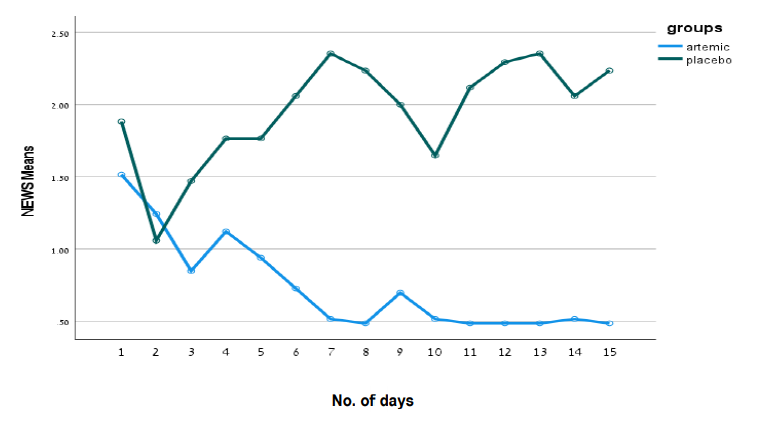
The Trial met all the FDA requirements for a COVID-19 study including population diversity (age, medical history, and genetic diversity) and demonstrated a full safety profile with no drug related adverse events. This resulted due to ArtemiCTM and the trial being focused on the immunomodulation specific for the prevention of cytokines storm, as opposed to other immunomodulators.
These results also follow safety and toxicity testing completed on mice and in line with FDA requirements for product registration requiring two types of rodents in pre-clinical trials. MGC Pharma completed an in vivo safety and toxicity pre-clinical study, including histology testing, on 24 rats. This included four groups with three study drug dosages being 48ug, 96ug and 196ug per kg rat and a control group.
The rats were observed and tested for clinical changes over seven days. This study included pathological examination of the organs: liver, heart, brain, spleen, spinal cord, sciatic nerve, kidney (L+R), lungs and tongue.
Further successful results from pre-clinical glioblastoma research
Results from the ongoing pre-clinical research program focused on evaluating cannabinoid formulations in the development of a treatment of the most aggressive and therapeutically resistant brain tumour, glioblastoma, have shown further successful results.
The pre-clinical in-vitro research program is being conducted in collaboration with the National Institute of Biology (‘NIB’) and the Neurosurgery Department at the University Medical Centre in Ljubljana, Slovenia.
The results from 18 patient tumour samples show for the first time that the Company’s proprietary formulation, CBG, exerts a superior effect in impairing the major hallmarks of glioblastoma progression, i.e. fast proliferation and invasion, and particularly enhancing glioblastoma cell death. Moreover, CBG can destroy therapy-resistant glioblastoma stem cells, which are the root of cancer development and extremely resistant to various treatments of this lethal cancer. CBG should present a new yet unexplored modality of glioblastoma therapy that could replace Tetrahydrocannabinol (THC) as a more acceptable add-on or adjuvant treatment strategy.
Financial and Corporate
Completion of unmarketable share parcel
The Company completed the sale of 50,696,634 fully paid ordinary shares pursuant to the Company’s Unmarketable Parcel Sale Facility (UMP Facility). MGC Pharma confirms its shareholder base has now been reduced by 5,067 shareholders. This will significantly reduce the Company’s administrative and corporate costs moving forward.
Progress towards completion of MGC Nutraceuticals sale to Onassis
As announced on 2nd December 2020, under the terms of the sale and purchase agreement for the 100% sale of the Company’s subsidiary MGC Nutraceuticals, MGC Pharma will receive shares equating to a value of US$6 million in Onassis Holdings Corp and the Company has secured an exclusive supply agreement for the provision of its CBD, raw materials and proprietary production intellectual property (IP). This follows the signing of a binding acquisition and exclusive CBD supply agreement as announced on 18 June 2020.
During the quarter MGC Pharma provided the 30 June 2020 audited financial statements for MGC Nutraceuticals to Onassis and full settlement of the acquisition is expected to complete over the next quarter. During the December quarter Onassis has commenced the process of finalising the offering submissions for the capital raising with the US SEC. The process from submission to completion is currently expected to complete in H1 2021. Once the offering submission has been approved, Onassis will complete the capital raising which will enable the full and complete settlement of the MGC Nutraceuticals acquisition with MGC Pharma.
Long terms benefits from UN vote to reschedule cannabis
In early December 2020, the United Nations (UN) voted in favour of the removal of cannabis and its derivatives from schedule IV in recommendations from the World Health Organisation (WHO). Cannabis and its derivativities are now contained under Schedule I of the 1961 UN Single Convention on Narcotic Drugs.
This creates a significant opportunity for MGC Pharma by removing red-tape that creates logistical limitations of the movement of products and creates an open pathway for easier and cheaper global distribution. This will also enable significantly more commercial opportunities for MGC Pharma by allowing it to deliver its Mercury Pharma product line to new markets going forward.
Appendix 4C
The Company had $1.57m cash at the end of the December 2020 quarter, with access to $9.25m undrawn from its $15m financing facility with Mercer Street Opportunity Fund LLC (as announced to the ASX on 10 September 2020). In accordance with Section 6 of the attached Appendix 4C, the Company confirms the total $480k was for executive director fees, non-executive director fees and corporate costs during the quarter.
As detailed in the Appendix 4C, expenditure for the quarter has been spent on $1.484m for research and development, $1.363m for manufacturing and operating costs, $152k for advertising and marketing, $258k staffing costs and $852k for administration and corporate costs.
saving nickels saving dimes
working till the sun don't shine
looking forward to happier times
1963, Roy Orbison - On Blue Bayou
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM







