Thursday, September 24, 2020 4:57:56 PM
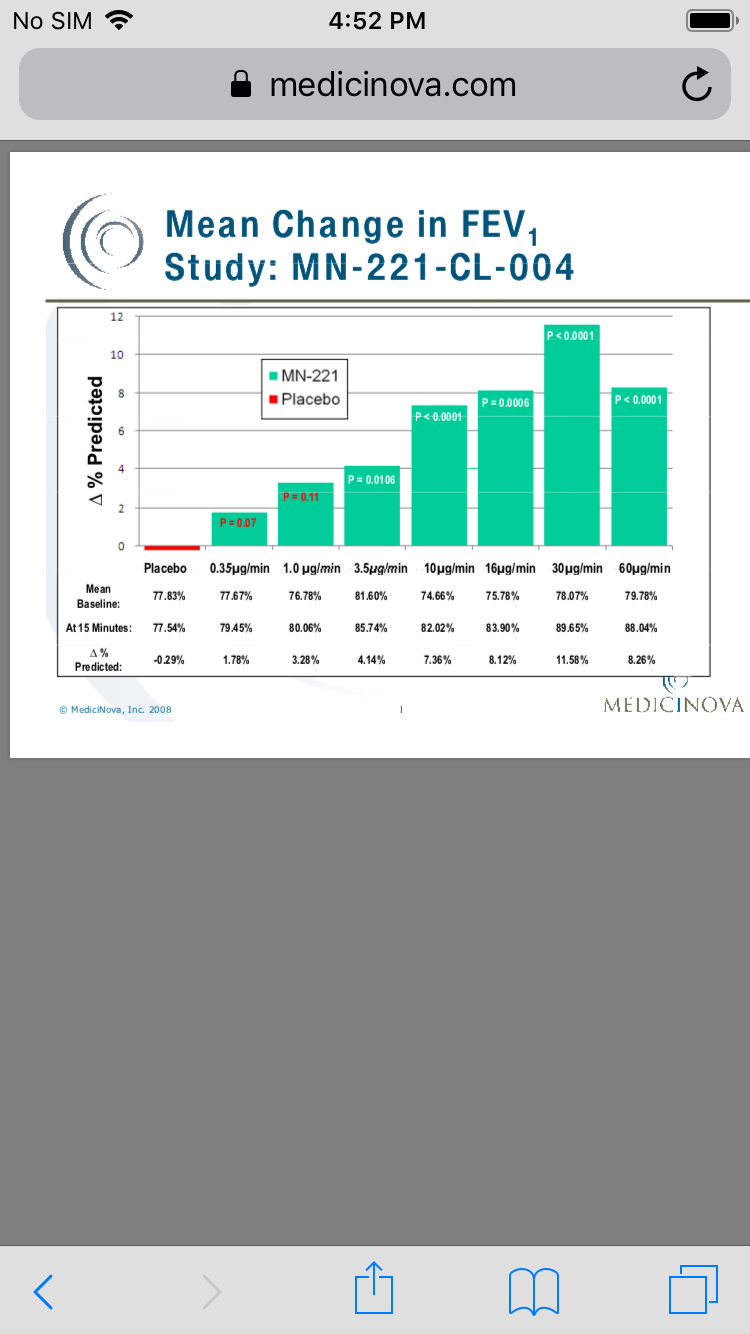
This clinical trial achieved statistical significance in its primary endpoint of mean change in forced expiratory volume in one second, or FEV1, from baseline to measurement at 15 minutes (the end of the infusion) at doses of 10, 16, 30 and 60 micrograms per minute of MN-221 (p-value less than or equal to 0.0006) compared to placebo.
There were no clinically significant cardiovascular, electrocardiogram, or ECG, or vital sign changes observed at any dose tested. In addition, no serious adverse effects were observed in this clinical trial.
Recent MNOV News
- MediciNova Announces Abstract Regarding MN-166 (ibudilast) in COMBAT-ALS Clinical Trial Accepted for Poster Presentation at the 35th International Symposium on ALS / MND • GlobeNewswire Inc. • 09/09/2024 11:00:00 PM
- MediciNova Announces Acceptance of Abstract Regarding MN-166 (ibudilast) in COMBAT-ALS Clinical Trial for Presentation at the 2024 Annual NEALS (Northeast Amyotrophic Lateral Sclerosis Consortium) Meeting • GlobeNewswire Inc. • 09/03/2024 11:00:00 PM
- MediciNova Receives Notice of Allowance from United States Patent and Trademark Office for New Patent Covering MN-166 (ibudilast) for the Post-COVID Condition • GlobeNewswire Inc. • 08/29/2024 11:00:00 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 08/08/2024 08:32:00 PM
- MediciNova Chief Business Officer David H. Crean, Ph.D. Assumes Communications Role Overseeing Investor Engagement and Public Relations • GlobeNewswire Inc. • 06/20/2024 10:00:00 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/12/2024 11:41:33 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/12/2024 11:40:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/12/2024 11:40:09 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 06/12/2024 08:15:10 PM
- MediciNova Receives Notice of Allowance for New Patent Covering MN-166 (ibudilast) for the Prevention of Metastasis of Various Solid Cancer • GlobeNewswire Inc. • 06/05/2024 10:00:00 AM
- MediciNova Announces Data from Phase 1b/2a Clinical Trial of MN-166 (ibudilast) in Glioblastoma Patients at the American Society of Clinical Oncology (ASCO) Annual Meeting 2024 • GlobeNewswire Inc. • 06/03/2024 10:00:00 AM
- MediciNova Announces Two Poster Presentations at the 92nd EAS Congress 2024, the Annual Meeting of the European Atherosclerosis Society Regarding the Use of MN-001 (Tipelukast) for Cardiometabolic Conditions • GlobeNewswire Inc. • 05/28/2024 10:00:00 AM
- MediciNova Receives Notice of Allowance for New Patent Covering MN-166 (ibudilast) for the Prevention of Metastasis of Eye Cancer • GlobeNewswire Inc. • 05/20/2024 10:00:00 AM
- MediciNova Receives Notice of Allowance for New Patent Covering MN-166 (ibudilast) for the Treatment of Chlorine-Induced Acute Respiratory Distress Syndrome • GlobeNewswire Inc. • 05/14/2024 10:00:00 AM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 05/09/2024 08:15:29 PM
- MediciNova Receives Issue Notification for New Patent Covering Extended-Release Formulations of MN-166 (ibudilast) • GlobeNewswire Inc. • 05/07/2024 10:00:00 AM
- MediciNova Announces Abstract Regarding Results of a Clinical Trial of MN-166 (ibudilast) in Glioblastoma Accepted for Presentation at the 2024 American Society of Clinical Oncology Annual Meeting (2024 ASCO) • GlobeNewswire Inc. • 04/02/2024 11:00:00 PM
- MediciNova Receives a Notice of Allowance for a New Patent Covering MN-166 (ibudilast) for the Treatment of Macular Injury in Japan • GlobeNewswire Inc. • 03/26/2024 11:00:00 PM
- MediciNova Announces Two Abstracts regarding MN-001 (tipelukast) and MN-002 Accepted for Presentation at the 92nd EAS 2024 Congress, the Annual Meeting of the European Atherosclerosis Society • GlobeNewswire Inc. • 03/20/2024 11:00:00 PM
- MediciNova Announces New Data and Results of MN-166 (ibudilast) in Chlorine Gas-induced Acute Lung Injury Presented at the 63rd Annual Meeting of the Society of Toxicology • GlobeNewswire Inc. • 03/12/2024 10:30:00 AM
- Form 10-K - Annual report [Section 13 and 15(d), not S-K Item 405] • Edgar (US Regulatory) • 02/15/2024 09:25:31 PM
- Form S-8 POS - Securities to be offered to employees in employee benefit plans, post-effective amendments • Edgar (US Regulatory) • 01/19/2024 09:27:28 PM
- Form S-8 POS - Securities to be offered to employees in employee benefit plans, post-effective amendments • Edgar (US Regulatory) • 01/19/2024 09:25:25 PM
- Form S-8 POS - Securities to be offered to employees in employee benefit plans, post-effective amendments • Edgar (US Regulatory) • 01/19/2024 09:23:50 PM
- Form S-8 POS - Securities to be offered to employees in employee benefit plans, post-effective amendments • Edgar (US Regulatory) • 01/19/2024 09:21:57 PM
FEATURED Cannabix Technologies and Omega Laboratories Inc. Advance Marijuana Breathalyzer Technology - Dr. Bruce Goldberger to Present at Society of Forensic Toxicologists Conference • Sep 24, 2024 8:50 AM
FEATURED Integrated Ventures, Inc Announces Strategic Partnership For GLP-1 (Semaglutide) Procurement Through MedWell USA, LLC. • Sep 24, 2024 8:45 AM
Avant Technologies Accelerates Creation of AI-Powered Platform to Revolutionize Patient Care • AVAI • Sep 24, 2024 8:00 AM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM






