| Followers | 2426 |
| Posts | 90823 |
| Boards Moderated | 7 |
| Alias Born | 09/12/2003 |
Friday, September 04, 2020 12:31:47 PM
UPGRADE OCQB
Select hospital locations U.S trials.
Sponsor for Trials
IND FDA Expanded Access Program (Compassionate Use) for Bucillamine in COVID-19
FDA Authorized emergency IND (eIND
Grant/Funding opportunities
Phase 3 clinical trials Bucillamine in the treatment of COVID-19 in the U.S
Expand Phase 3 Clinical Trials COVID-19 in Asia-Pacific and Canada
Q2 Submit IND for Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
Q2/3 Pre-IND meeting with FDA for Bucillamine in various infectious diseases (undisclosed)
Update the final Prototype orally dissolvable thin film strip for psilocybin
Patents approval process psilocybin formulations
Finalize vendor agreements in project management, medical monitoring, and data management.
Patent approval News Updates:
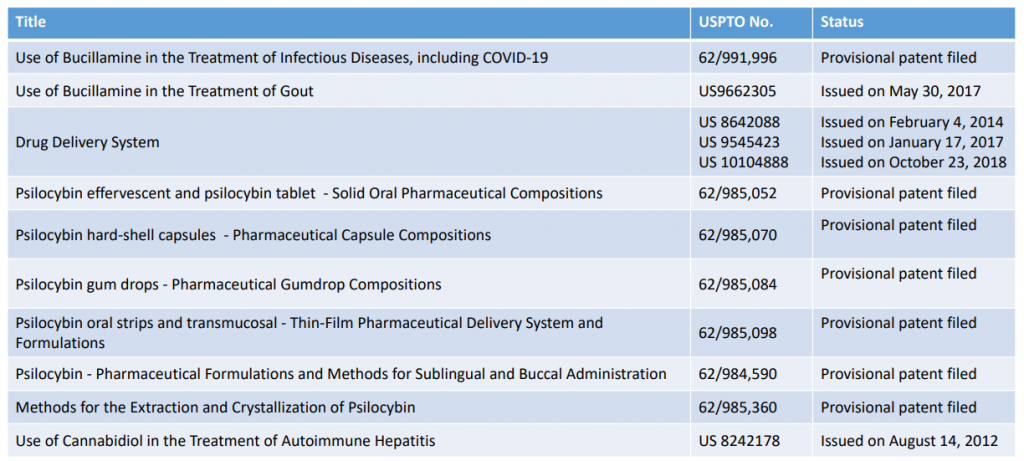
Q3 Initiate Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
Q3 Pre-IND meeting with FDA for Psilocybin (undisclosed indications)
Q3/4 Results from Phase 3 clinical study of Bucillamine in the treatment of COVID-19
Q4 Results from Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
Potential buyout could be Billions for a biotech company
Manufacturing deal Atwill Medical Solutions CEO is on the Revive board just signed a deal with VXRT This could lead to potential partnership/deals in the future. IMHO:))
Update on its Psilocybin-Based Pharmaceutical Program
Partners to Advance Research for Psilocybin-Based Pharmaceutical Products
Update drugs research partnership agreement (“SRPA”) with the University of Wisconsin-Madison
Link to Revive's subsidiary company
https://www.psilocinpharma.com/
VAYK Discloses Strategic Conversation on Potential Acquisition of $4 Million Home Service Business • VAYK • May 9, 2024 9:00 AM
Bantec's Howco Awarded $4.19 Million Dollar U.S. Department of Defense Contract • BANT • May 8, 2024 10:00 AM
Element79 Gold Corp Successfully Closes Maverick Springs Option Agreement • ELEM • May 8, 2024 9:05 AM
Kona Gold Beverages, Inc. Achieves April Revenues Exceeding $586,000 • KGKG • May 8, 2024 8:30 AM
Epazz plans to spin off Galaxy Batteries Inc. • EPAZ • May 8, 2024 7:05 AM
Moon Equity Holdings, Corp. Announces Acquisition of Wikolo, Inc. • MONI • May 7, 2024 9:48 AM










