| Followers | 2429 |
| Posts | 91540 |
| Boards Moderated | 3 |
| Alias Born | 09/12/2003 |
Sunday, August 02, 2020 1:31:43 PM
****UPDATE: REVIVE THERAPEUTIC FUNDAMENTAL DD...****
The Shocking Truth About Bucillamine is already being manufactured/produced, all that needs to happen is it get approved for COVID-19 treatment. Bucillamine know no toxicity related to drug. In light of this I honestly believe three to six months tops until it's approved and on its way to market. It is important that you understand, you be aware of all the changes that company making. Therefore, I urge you to see it once June Investor presentation. They have their highest market cap comparison at 3.7 billion that equal to $15 dollars per share. In my humble opinion I will tell you this is only the beginning! There will be a lot more exciting NEWS coming. I'm looking forward to see they announce sponsor/partnership, funding...NEAR TERM CATALYST I list at the end of my DD page. Enjoy to read my DD.

For more information, visit https://www.revivethera.com/
BREAKING NEWS:
UPDATE - Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19
https://finance.yahoo.com/news/revive-therapeutics-announces-u-fda-142700139.html
Pharmaceutical Product PIPELINE
Revive is building a pharmaceutical product portfolio targeting rare disorders and infectious diseases.

THE TOP COMPANIES RACING TO PRODUCE PANDEMIC TREATMENTS:
https://stockhouse.com/news/press-releases/2020/07/31/these-are-some-of-the-top-companies-racing-to-produce-pandemic-treatments
Investor Deck - JUNE 2020
https://www.revivethera.com/uploads/1/0/1/0/101019330/rvv_deck_-_june202023.pdf
RVVTF could be the FIRST the only one get the approval by FDA for use treatment in Covid-19
Coronavirus Treatment Acceleration Program (CTAP)
510+ Drug development program in planning stages. 230+ trials reviews by FDA
Only 2 Covid 19 treatments currently authorized for Emergency use. ZERO TREATMENT currently approved by FDA for used in Covid-19
https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
Revive Therapeutics LIST WITH THE BIG-BOYS top manufacturers/players such as Bristol-Myers, Gilead Lifesciences, Pfizer Inc...
https://3wnews.org/industry-reports/2933080/autoimmune-hepatitis-diagnosis-and-treatment-market-pin-point-analysis-and-future-strategies-by-2026-opportunities-forecast/
BIOPHARMCATALYST DATA BASE: Scroll down, you can see where RVVTF is listed and the site gives updates on what phase it is in and when it is supposed to be complete.
https://www.biopharmcatalyst.com/biotech-stocks/company-pipeline-database
CYDY O/S 564 Million 3 times higher than RVVTF (both) stock list on OCT. CYDY move from .35 to $10.00 on phase2 trial. It only makes sense to see us run past that ten dollar mark.
UPDATE - Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19
https://finance.yahoo.com/news/revive-therapeutics-announces-u-fda-142700139.html
Expected PPS do well when Health Canada approval for Phase3 trials this could happen at any time.
Revive Therapeutics Provides Update on Discussions with Health Canada in Pre-CTA Meeting
https://finance.yahoo.com/news/revive-therapeutics-provides-discussions-health-160632558.html
Revive Therapeutics CSE: R V V) (OTCQB RVVTF)ADVERTISED ON BLOOMBERG TV, IN CANADA…
https://www.b-tv.com/all-videos/revive-therapeutics-for-rare-disorders-and-infectious-diseases-commercial-15sec
Check it out! Revive Employee Dr Mpunjub Is Ex FDA Drug Reviewer:
https://www.revivethera.com/clinical-team.html
AMAZING VIDEO! RVVTF being compared to VXRT and CYDY ==>
https://www.proactiveinvestors.co.uk/companies/news/923054/revive-
RVVTF Revive Therapeutics Director Signs Manufacturing Arrangement With Vaxart
https://thedeepdive.ca/revive-therapeutics-director-signs-manufacturing-arrangement-with-vaxart/
A Study of N-acetylcysteine in Patients With COVID-19 Infection. The study researchers think that a medication called N-acetylcysteine can help fight the COVID-19 virus by boosting a type of cell in your immune system that attacks infections. View link bellow:
A Study of N-acetylcysteine in Patients With COVID-19 Infection
https://clinicaltrials.gov/ct2/show/study/NCT04374461
Bucillamine, a cysteine derivative with two thiol groups, has been shown to be 16 times more potent as a thiol donor in vivo than NAC. (N-Acetyl Cysteine (Nac) It's no wonder the FDA gave it the fast track go ahead into phase 3.
A must watch video: Analyst sees 'substantial upside potential' as key assets move towards clinic
NEAR TERM CATALYST :
1 * Initiate Phase 3 clinical study of Bucillamine in the treatment of COVID-19 in the U.S
2. Asia updates on trials for Covid-19 Bucillamine
3. Canadian updates on Covid-19 Bucillamine
4. Q2 Submit IND for Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
5 * Q2/3 Pre-IND meeting with FDA for Bucillamine in various infectious diseases (undisclosed)
6 * Q3 Initiate Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
7. Q3 Pre-IND meeting with FDA for Psilocybin (undisclosed indications)
8 * Q3/4 Results from Phase 2 clinical study of Bucillamine in the treatment of COVID-19
9 * Q4 Results from Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
10 * Q4 Submit FDA IND for Phase 2 clinical study of Bucillamine (undisclosed indication)
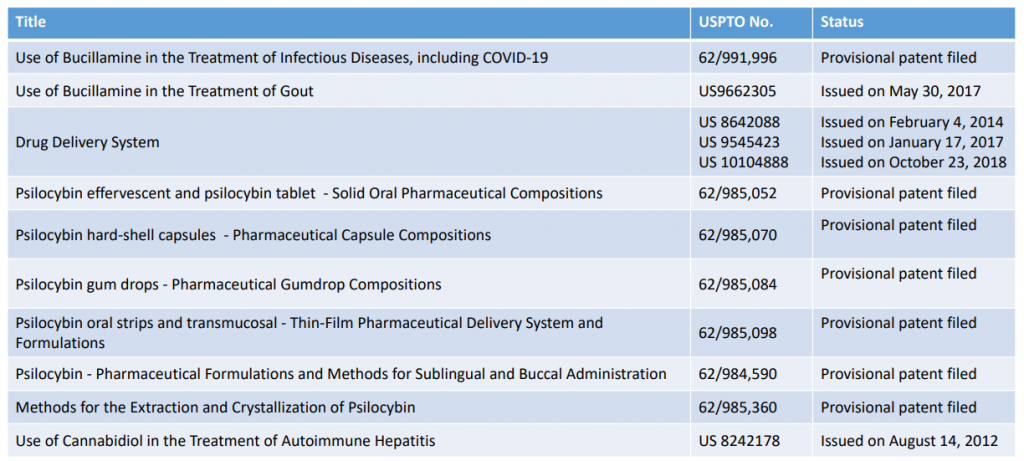
11 * Patent News Updates
12 * Sponsor/Partnership for Trials
13 * Funding
14 * Potential buyout could be Billions for a biotech company
16. Manufacturing deal Atwill Medical Solutions CEO is on the Revive board just signed a deal with VXRT This could lead to potential partnership/deals in the future. IMHO:))
Vaxart, Inc. Signs Memorandum of Understanding with Attwill Medical Solutions Sterilflow, LP?William Jackson, Director on Revive board
https://www.revivethera.com/board-of-directors.html
Revive has an exclusive license from South Carolina Research Foundation for its intellectual property for the use of CBD. (U.S. patent No. 8242178). Also, the FDA has granted to Revive orphan drug designation for CBD in the treatment of AIH.
LATEST PRESS RELEASES:
https://www.revivethera.com/

The Shocking Truth About Bucillamine is already being manufactured/produced, all that needs to happen is it get approved for COVID-19 treatment. Bucillamine know no toxicity related to drug. In light of this I honestly believe three to six months tops until it's approved and on its way to market. It is important that you understand, you be aware of all the changes that company making. Therefore, I urge you to see it once June Investor presentation. They have their highest market cap comparison at 3.7 billion that equal to $15 dollars per share. In my humble opinion I will tell you this is only the beginning! There will be a lot more exciting NEWS coming. I'm looking forward to see they announce sponsor/partnership, funding...NEAR TERM CATALYST I list at the end of my DD page. Enjoy to read my DD.

For more information, visit https://www.revivethera.com/
BREAKING NEWS:
UPDATE - Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19
https://finance.yahoo.com/news/revive-therapeutics-announces-u-fda-142700139.html
Pharmaceutical Product PIPELINE
Revive is building a pharmaceutical product portfolio targeting rare disorders and infectious diseases.

THE TOP COMPANIES RACING TO PRODUCE PANDEMIC TREATMENTS:
https://stockhouse.com/news/press-releases/2020/07/31/these-are-some-of-the-top-companies-racing-to-produce-pandemic-treatments
Investor Deck - JUNE 2020
https://www.revivethera.com/uploads/1/0/1/0/101019330/rvv_deck_-_june202023.pdf
RVVTF could be the FIRST the only one get the approval by FDA for use treatment in Covid-19
Coronavirus Treatment Acceleration Program (CTAP)
510+ Drug development program in planning stages. 230+ trials reviews by FDA
Only 2 Covid 19 treatments currently authorized for Emergency use. ZERO TREATMENT currently approved by FDA for used in Covid-19
https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
Revive Therapeutics LIST WITH THE BIG-BOYS top manufacturers/players such as Bristol-Myers, Gilead Lifesciences, Pfizer Inc...
https://3wnews.org/industry-reports/2933080/autoimmune-hepatitis-diagnosis-and-treatment-market-pin-point-analysis-and-future-strategies-by-2026-opportunities-forecast/
BIOPHARMCATALYST DATA BASE: Scroll down, you can see where RVVTF is listed and the site gives updates on what phase it is in and when it is supposed to be complete.
https://www.biopharmcatalyst.com/biotech-stocks/company-pipeline-database
CYDY O/S 564 Million 3 times higher than RVVTF (both) stock list on OCT. CYDY move from .35 to $10.00 on phase2 trial. It only makes sense to see us run past that ten dollar mark.
UPDATE - Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19
https://finance.yahoo.com/news/revive-therapeutics-announces-u-fda-142700139.html
Expected PPS do well when Health Canada approval for Phase3 trials this could happen at any time.
Revive Therapeutics Provides Update on Discussions with Health Canada in Pre-CTA Meeting
https://finance.yahoo.com/news/revive-therapeutics-provides-discussions-health-160632558.html
Revive Therapeutics CSE: R V V) (OTCQB RVVTF)ADVERTISED ON BLOOMBERG TV, IN CANADA…
https://www.b-tv.com/all-videos/revive-therapeutics-for-rare-disorders-and-infectious-diseases-commercial-15sec
Check it out! Revive Employee Dr Mpunjub Is Ex FDA Drug Reviewer:
https://www.revivethera.com/clinical-team.html
AMAZING VIDEO! RVVTF being compared to VXRT and CYDY ==>
https://www.proactiveinvestors.co.uk/companies/news/923054/revive-
RVVTF Revive Therapeutics Director Signs Manufacturing Arrangement With Vaxart
https://thedeepdive.ca/revive-therapeutics-director-signs-manufacturing-arrangement-with-vaxart/
A Study of N-acetylcysteine in Patients With COVID-19 Infection. The study researchers think that a medication called N-acetylcysteine can help fight the COVID-19 virus by boosting a type of cell in your immune system that attacks infections. View link bellow:
A Study of N-acetylcysteine in Patients With COVID-19 Infection
https://clinicaltrials.gov/ct2/show/study/NCT04374461
Bucillamine, a cysteine derivative with two thiol groups, has been shown to be 16 times more potent as a thiol donor in vivo than NAC. (N-Acetyl Cysteine (Nac) It's no wonder the FDA gave it the fast track go ahead into phase 3.
A must watch video: Analyst sees 'substantial upside potential' as key assets move towards clinic
NEAR TERM CATALYST :
1 * Initiate Phase 3 clinical study of Bucillamine in the treatment of COVID-19 in the U.S
2. Asia updates on trials for Covid-19 Bucillamine
3. Canadian updates on Covid-19 Bucillamine
4. Q2 Submit IND for Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
5 * Q2/3 Pre-IND meeting with FDA for Bucillamine in various infectious diseases (undisclosed)
6 * Q3 Initiate Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
7. Q3 Pre-IND meeting with FDA for Psilocybin (undisclosed indications)
8 * Q3/4 Results from Phase 2 clinical study of Bucillamine in the treatment of COVID-19
9 * Q4 Results from Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
10 * Q4 Submit FDA IND for Phase 2 clinical study of Bucillamine (undisclosed indication)
11 * Patent News Updates
12 * Sponsor/Partnership for Trials
13 * Funding
14 * Potential buyout could be Billions for a biotech company
16. Manufacturing deal Atwill Medical Solutions CEO is on the Revive board just signed a deal with VXRT This could lead to potential partnership/deals in the future. IMHO:))
Vaxart, Inc. Signs Memorandum of Understanding with Attwill Medical Solutions Sterilflow, LP?William Jackson, Director on Revive board
https://www.revivethera.com/board-of-directors.html
Revive has an exclusive license from South Carolina Research Foundation for its intellectual property for the use of CBD. (U.S. patent No. 8242178). Also, the FDA has granted to Revive orphan drug designation for CBD in the treatment of AIH.

LATEST PRESS RELEASES:
https://www.revivethera.com/
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.







