Tuesday, July 07, 2020 12:32:21 PM
by Magnus Schroeder, PhD, VP/ProcessDev, Avid Bioservices
https://www.pharmasalmanac.com/articles/accelerating-covid-19-programs-with-comprehensive-support-from-cell-line-to-commercial-production-at-a-single-site
Excerpt:
“In March 2020, Avid Bioservices was honored to receive 2020 Contract Manufacturing Organization (CMO) Leadership Awards (https://tinyurl.com/yb5cnh6m ) for Capabilities, Expertise, Reliability, Compatibility, and Service based on market research and surveys conducted by Industry Std. Research (ISR) and presented by industry publication Life Science Leader. We were also named a 2020 CMO Leadership Award Champion in the categories of Expertise & Service.
These awards are truly gratifying, because Avid’s entire business philosophy is built upon the concept of doing whatever it takes to deliver for our customers. We work to an incredibly high standard, with our teams taking real ownership of client programs and treating them as if they were internal projects.
They also underscore the key attributes of Avid as a CDMO well-positioned to help companies responding to the COVID-19 pandemic. We can support the process development and manufacture of traditional and novel potential therapies. We can rapidly advance candidates all the way to commercialization from a single site, eliminating complex and time-consuming site transfers…”

= = = = = =
5-18-20: Avid Listed In Top CMO Awards Article https://tinyurl.com/yb5cnh6m
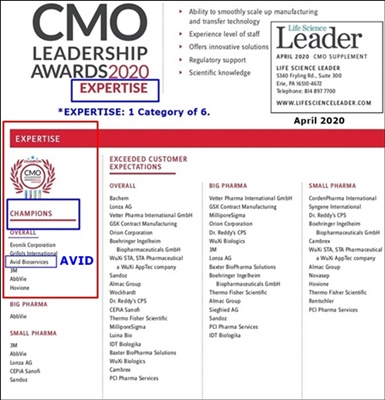
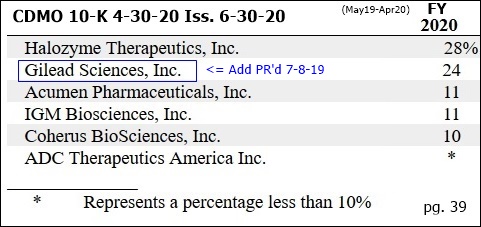
7-8-19/PR: Avid Signs New Top10 Global Pharma [GILEAD] https://tinyurl.com/yyq8zgb9
7-3-20/BioProcINTL: “Avid Preps To Kick-Off $30-40m Mfg. Expansion”
by Dan Stanton (Editor - BioProcess Insider, part of Informa Life Sciences' BioProcess Intl.)
https://bioprocessintl.com/bioprocess-insider/facilities-capacity/avid-preps-to-kick-off-30-40m-manufacturing-expansion/
= = = = = = =6-30-20 CC re: cv19 https://tinyurl.com/y9zbgmos
6-30-20: Joe Pantginis (H.C. Wainwright), “...going back to the potential COVID contracts…”
RICK HANCOCK (Int. CEO): So the contracts we're looking at currently, exclusivity is not really a part of it. The main drivers to get something through proof of concept as quickly as possible and I think one of the things that makes Avid an attractive partner for these kinds of things is, while we don't have the largest scale, some of them are requiring very small doses or very small amounts of material per dose, but the fact that we could take something very rapidly from proof of concept to commercial production with our many years of commercial capability. Just my opinion, I think the strategy of most of the people that we're talking to, based on where they are getting their funding from, the goal is to identify something that works either as a vaccine or therapeutic and then make it as widely available as possible.
TIM COMPTON (COO): It's very much timeline driven and certainly the exclusivity in those types of legal agreements have not been engaged or discussed on. It's really about delivering and timeline to deliver.
6-30-20: Matt Hewitt (Craig-Hallum), ”but given these COVID opportunities, how quickly would you anticipate hearing back…”
TIM COMPTON (COO): ...every opportunity is different, every timeline is different, but we certainly anticipate hearing back from some of the opportunities that we have in the queue for COVID probably in the next - probably next 90 days, but there are several opportunities in various stages of the sales cycle.
Recent CDMO News
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:33:03 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:32:11 AM
FEATURED NanoViricides Reports that the Phase I NV-387 Clinical Trial is Completed Successfully and Data Lock is Expected Soon • May 2, 2024 10:07 AM
ILUS Files Form 10-K and Provides Shareholder Update • ILUS • May 2, 2024 8:52 AM
Avant Technologies Names New CEO Following Acquisition of Healthcare Technology and Data Integration Firm • AVAI • May 2, 2024 8:00 AM
Bantec Engaged in a Letter of Intent to Acquire a Small New Jersey Based Manufacturing Company • BANT • May 1, 2024 10:00 AM
Cannabix Technologies to Deliver Breath Logix Alcohol Screening Device to Australia • BLO • Apr 30, 2024 8:53 AM
Hydromer, Inc. Reports Preliminary Unaudited Financial Results for First Quarter 2024 • HYDI • Apr 29, 2024 9:10 AM










