Friday, March 23, 2018 9:34:13 AM
$CELZ Low Float Stem Cell. Next mega stem cell runner.. USRM version 2.0.
Share Structure Information As Of 3-2-18
Authorized Shares - 3,000,000,000
Outstanding Shares - 233,571,295
52-Wk Range
0.0021 - 0.70
https://www.otcmarkets.com/stock/CELZ/overview
PHOENIX, Feb. 20, 2018 /PRNewswire -- Creative Medical Technology Holdings (OTCQB:CELZ) announced today the formation of CerebroStem LLC, a majority owned subsidiary focused on developing stem cell therapies for brain injuries and neurodegenerative diseases. The Company's initial focus will be treating radiation induced brain damage, a major cause of cognitive dysfunction in patients with brain cancer who have received radiation therapy.
"The AmnioStem(TM) amniotic stem cell product offers a potent platform for developing second generation stem cell therapies useful for neurodegenerative and other neurological diseases," said Santosh Kesari, MD, Ph.D, FANA, FAAN, Chair and Professor, Department of Translational Neurosciences and Neurotherapeutics, John Wayne Cancer Institute, as well as Director of Neuro-Oncology, Providence Saint John's Health Center, who leads the Pacific Neuroscience Research Center at Pacific Neuroscience Institute. "I am enthusiastic to combine new treatments and procedures developed by AmnioStem(TM) universal donor cell therapy, which I believe will allow for rapid clinical entry."
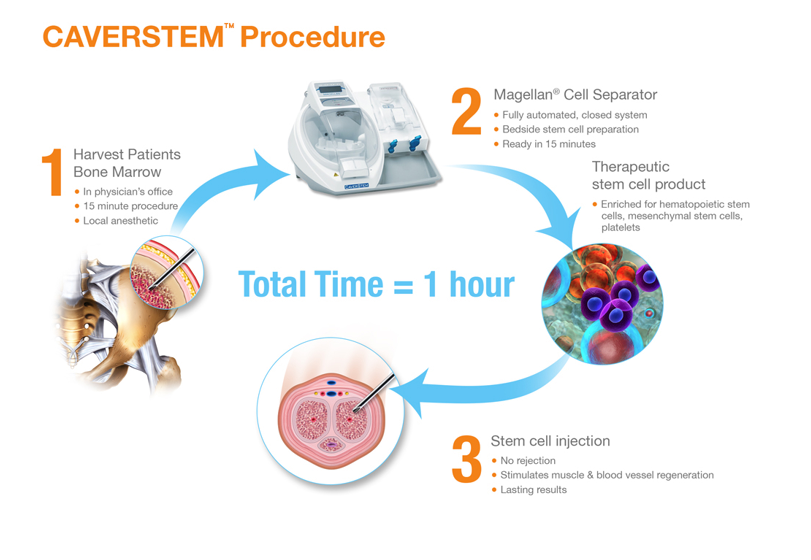
PHOENIX, and LOS ANGELES, Dec. 18, 2017 /PRNewswire/ -- Creative Medical Technology Holdings, Inc. (OTCQB ticker symbol CELZ) announced today initiation of patient treatments using its patented Caverstem™ procedure by Dr. Alexander Gershman.
Dr. Alexander Gershman, Director of The Institute of Advanced Urology at the Cedars-Sinai Medical Tower; Director of Urologic Laparoscopy in the Division of Urology, Harbor-UCLA Medical Center, began treating patients with the procedure, which involves extraction of a small amount of bone marrow, concentration of bone marrow derived stem cells, and subsequent administration to the patient within less than an hour.
"There is much excitement about stem cells revolutionizing many aspects of medicine. I am enthusiastic to be offering this cutting edge procedure to my patients, for which previous therapies have failed or are too invasive," said Dr. Gershman. "Numerous publications and a recent clinical trial at the Los Angeles Biomedical Institute at Harbor – UCLA Hospital in Torrance, CA, support the safety and feasibility of the Caverstem™ procedure.
The biological basis of the bone marrow based treatment is that in patients with drug non-responsive erectile dysfunction, there is a deficit in function of smooth muscle and blood vessel cells. By administering the patient's own stem cells we aim to rebuild these damaged tissues, and thereby restore erectile function."
PHOENIX, Oct. 19, 2017 /PRNewswire/ -- Creative Medical Technology Holdings (OTCQB – CELZ) announced today completion of the safety data analysis on 20 patients with pharmacologically-resistant erectile dysfunction treated with the Company's patented CaverStem™ procedure.
The trial, sponsored by Creative Medical Technology Holdings, was conducted at the University of California Los Angeles Harbor Hospital/LA Biomed under Institutional Review Board (IRB) approval. An independent medical safety monitor was also appointed to review the patient data for safety and feasibility of administering bone marrow derived stem cells into patients with erectile dysfunction. The goal of this procedure is to regenerate blood vessels and smooth muscle, parts of the penis that are not functioning properly in this patient population.
"Based on establishment of safety of the CaverStem™ procedure in a formal university-based clinical trial, and independent confirmation of efficacy in an European clinical trial, we have launched commercialization for the CaverStem™ procedure," said Timothy Warbington, President and Chief Executive Officer of Creative Medical Technology Holdings. "Amongst other top urologists, we have recruited a world-renowned urologist as a lead physician to roll-out the procedure. We anticipate that the procedure will be available to patients that meet the eligibility criteria within the next 60 days."
PHOENIX and SAN DIEGO, May 24, 2017 /PRNewswire/ -- Creative Medical Technology Holdings, Inc. (OTCQB: CELZ) (the "Company") announced today the filing of a patent application covering a novel means of suppressing inflammation associated with stroke by reprogramming immune cells of the stroke victim.
The patent application teaches ways of using the Company's patented AmnioStem™ allogeneic stem cell to 'educate' immune cells from stroke patients so as to reduce inflammation associated with stroke. By reducing inflammation, the Company hopes to develop novel means of increasing efficacy of therapeutic agents in stroke patients.



Previous | Next
Share Structure Information As Of 3-2-18
Authorized Shares - 3,000,000,000
Outstanding Shares - 233,571,295
52-Wk Range
0.0021 - 0.70
https://www.otcmarkets.com/stock/CELZ/overview
PHOENIX, Feb. 20, 2018 /PRNewswire -- Creative Medical Technology Holdings (OTCQB:CELZ) announced today the formation of CerebroStem LLC, a majority owned subsidiary focused on developing stem cell therapies for brain injuries and neurodegenerative diseases. The Company's initial focus will be treating radiation induced brain damage, a major cause of cognitive dysfunction in patients with brain cancer who have received radiation therapy.
"The AmnioStem(TM) amniotic stem cell product offers a potent platform for developing second generation stem cell therapies useful for neurodegenerative and other neurological diseases," said Santosh Kesari, MD, Ph.D, FANA, FAAN, Chair and Professor, Department of Translational Neurosciences and Neurotherapeutics, John Wayne Cancer Institute, as well as Director of Neuro-Oncology, Providence Saint John's Health Center, who leads the Pacific Neuroscience Research Center at Pacific Neuroscience Institute. "I am enthusiastic to combine new treatments and procedures developed by AmnioStem(TM) universal donor cell therapy, which I believe will allow for rapid clinical entry."
PHOENIX, and LOS ANGELES, Dec. 18, 2017 /PRNewswire/ -- Creative Medical Technology Holdings, Inc. (OTCQB ticker symbol CELZ) announced today initiation of patient treatments using its patented Caverstem™ procedure by Dr. Alexander Gershman.
Dr. Alexander Gershman, Director of The Institute of Advanced Urology at the Cedars-Sinai Medical Tower; Director of Urologic Laparoscopy in the Division of Urology, Harbor-UCLA Medical Center, began treating patients with the procedure, which involves extraction of a small amount of bone marrow, concentration of bone marrow derived stem cells, and subsequent administration to the patient within less than an hour.
"There is much excitement about stem cells revolutionizing many aspects of medicine. I am enthusiastic to be offering this cutting edge procedure to my patients, for which previous therapies have failed or are too invasive," said Dr. Gershman. "Numerous publications and a recent clinical trial at the Los Angeles Biomedical Institute at Harbor – UCLA Hospital in Torrance, CA, support the safety and feasibility of the Caverstem™ procedure.
The biological basis of the bone marrow based treatment is that in patients with drug non-responsive erectile dysfunction, there is a deficit in function of smooth muscle and blood vessel cells. By administering the patient's own stem cells we aim to rebuild these damaged tissues, and thereby restore erectile function."
PHOENIX, Oct. 19, 2017 /PRNewswire/ -- Creative Medical Technology Holdings (OTCQB – CELZ) announced today completion of the safety data analysis on 20 patients with pharmacologically-resistant erectile dysfunction treated with the Company's patented CaverStem™ procedure.
The trial, sponsored by Creative Medical Technology Holdings, was conducted at the University of California Los Angeles Harbor Hospital/LA Biomed under Institutional Review Board (IRB) approval. An independent medical safety monitor was also appointed to review the patient data for safety and feasibility of administering bone marrow derived stem cells into patients with erectile dysfunction. The goal of this procedure is to regenerate blood vessels and smooth muscle, parts of the penis that are not functioning properly in this patient population.
"Based on establishment of safety of the CaverStem™ procedure in a formal university-based clinical trial, and independent confirmation of efficacy in an European clinical trial, we have launched commercialization for the CaverStem™ procedure," said Timothy Warbington, President and Chief Executive Officer of Creative Medical Technology Holdings. "Amongst other top urologists, we have recruited a world-renowned urologist as a lead physician to roll-out the procedure. We anticipate that the procedure will be available to patients that meet the eligibility criteria within the next 60 days."
PHOENIX and SAN DIEGO, May 24, 2017 /PRNewswire/ -- Creative Medical Technology Holdings, Inc. (OTCQB: CELZ) (the "Company") announced today the filing of a patent application covering a novel means of suppressing inflammation associated with stroke by reprogramming immune cells of the stroke victim.
The patent application teaches ways of using the Company's patented AmnioStem™ allogeneic stem cell to 'educate' immune cells from stroke patients so as to reduce inflammation associated with stroke. By reducing inflammation, the Company hopes to develop novel means of increasing efficacy of therapeutic agents in stroke patients.
Previous | Next
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.







