Wednesday, December 13, 2017 8:01:32 AM
Shares Outstanding 5 152.67M
Float 83.12M
% Held by Insiders 1 65.59%
https://finance.yahoo.com/quote/OBMP?p=OBMP
Filings
https://www.sec.gov/cgi-bin/browse-edgar?action=getcompany&CIK=0001362703&type=&dateb=&owner=include&count=40
Press releases
http://oncbiomune.com/news/
Pipeline:
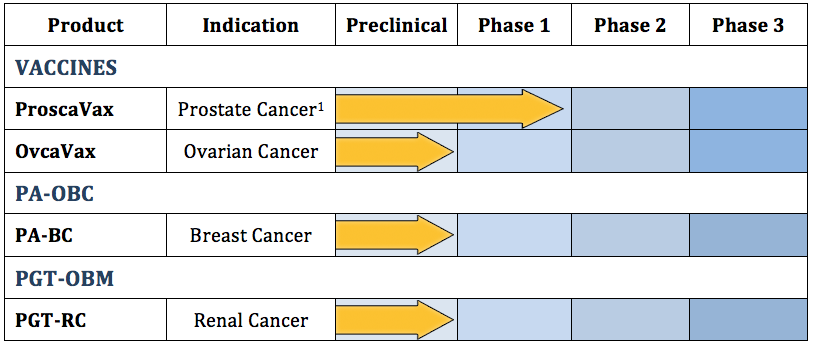
100% of Prostate Cancer Patients Completing 31-Week Post-Therapy Exam After Treatment with OncBioMune Immunotherapy Show No Disease Progression
BATON ROUGE, La., Dec. 05, 2017 — OncBioMune Pharmaceuticals, Inc. (OTCQB:OBMP) (“OncBioMune” or the “Company”), a revenue-stage biopharmaceutical company engaged in the development of a proprietary immunotherapy cancer vaccine technology, targeted cancer therapies and commercialization of a portfolio of products internationally, is pleased to provide the latest data from the Company’s successfully completed Phase 1 trial of ProscaVax for prostate cancer, suggesting a durable response 31 weeks post-therapy.
In the Phase 1 clinical trial, hormone-naïve and hormone-independent recurrent prostate cancer patients with increasing prostate specific antigen (PSA) were treated with six intradermal injections of ProscaVax. ProscaVax is OncBioMune’s novel immunotherapeutic cancer vaccine consisting of a combination of prostate cancer associated PSA with the biological adjuvants interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF).
As previously reported, all 20 patients enrolled in the trial completed ProscaVax therapy per protocol and completed the first follow-up at 19 weeks, with 16 of 20 patients (80%) demonstrating stable disease/no prostate cancer progression. Only four patients progressed during ProscaVax therapy (3 PSA progression and radiological progression in one patient with primary brain cancer who died from the primary disease).
15 of 16 patients with stable disease at 19 weeks have now completed additional follow-up examinations 31 weeks post-therapy. All 15 patients continued to live with stable disease / no prostate cancer progression. One patient with stable disease at 19 weeks did not complete the 31-week post-therapy visit. OncBioMune has been informed that individual enrolled in a separate clinical study, but no information on any prostate cancer progression has been reported to the Company.
Data continues to confirm there were no drug-related serious adverse events or dose-limiting toxicities resulting from ProscaVax therapy.
http://oncbiomune.com/2017/12/05/100-of-prostate-cancer-patients-completing-31-week-post-therapy-exam-after-treatment-with-oncbiomune-immunotherapy-show-no-disease-progression/
=====================
OncBioMune Awaits Approval from Regulatory Committee to Commence Phase 2 Trial of ProscaVax as Front Line Prostate Cancer Treatment
BATON ROUGE, LA–(August 31, 2017) – OncBioMune Pharmaceuticals, Inc. (OTCQB: OBMP)(“OncBioMune” or the “Company”), a revenue-stage biopharmaceutical company engaged in the development of targeted cancer therapies, a proprietary cancer vaccine technology and commercialization of a portfolio of products internationally, is pleased to report that the protocol for the planned Phase 2 study of ProscaVax for early-stage prostate cancer is now under review by the Regulatory Committee at Beth Israel Deaconess Medical Center/Harvard Medical School, the host hospital network for the study.
In the trial, ProscaVax, OncBioMune’s novel immunotherapeutic cancer vaccine consisting of a combination of tumor-associated prostate specific antigen (PSA) with the biological adjuvants interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF), will be evaluated for safety, tolerability and efficacy in treatment-naïve prostate cancer patients at disease presentation. Per the protocol, patients to be enrolled in the study will be newly diagnosed with prostate cancer, deciding in collaboration with their oncologist to forego standard approved therapies, such as a radical prostatectomy, radiation or brachytherapy, in favor of careful monitoring for disease progression, a process known more commonly as “active surveillance.” Presently, there are no FDA-approved treatments for prostate cancer patients in active surveillance.
“The Regulatory Committee is a component of the Institutional Review Board and integral to the protocol approval process to commence the study,” commented Dr. Jonathan Head, Chief Executive Officer at OncBioMune. “I interpret the protocol now being in their hands as a signal that we are getting close to conducting the first ever trial of ProscaVax as a front-line treatment for prostate cancer.”
http://oncbiomune.com/2017/08/31/oncbiomune-awaits-approval-from-regulatory-committee-to-commence-phase-2-trial-of-proscavax-as-front-line-prostate-cancer-treatment/
As submitted, the Phase 2 clinical protocol will randomly enroll 120 early stage prostate cancer patients with 40 patients on standard active surveillance and 80 patients receiving ProscaVax. The patients’ PSAs and yearly biopsies will be monitored for disease progression. The follow-up of the patients will be for two years. The investigators are Dr. Rupal Bhatt, Dr. Glenn Bubley and Dr. David Einstein at Beth Israel Deaconess Medical Center of Harvard Medical School. Early clinical research of ProscaVax, including a Phase 1 Clinical Trial that recently completed enrollment in PSA progressing prostate cancer in hormone-naïve and hormone-independent patients, has shown ProscaVax to be well tolerated with a strong safety profile. Data from late-stage patients further shows signs of efficacy with respect to an increased immune response and slowing tumor progression.
http://oncbiomune.com/2017/06/01/protocol-submitted-to-fda-for-phase-2-clinical-trial-of-proscavax-as-immunotherapy-for-early-stage-prostate-cancer/
================================
Enrollment to Begin in OncBioMune’s Trial of ProscaVax for Prostate Cancer in Mexico
http://oncbiomune.com/2017/06/27/enrollment-to-begin-in-oncbiomunes-trial-of-proscavax-for-prostate-cancer-in-mexico/
In the study, OncBioMune’s immunotherapeutic vaccine, ProscaVax, will be evaluated in prostate specific antigen (PSA) recurrent prostate cancer in hormone-naïve and hormone-independent patients. The trial is scheduled to enroll 50 patients in the Phase 2 portion. Patients will receive six vaccines of ProscaVax, a combination of the tumor-associated antigen PSA with the biological adjuvants IL-2 and GM-CSF. The Phase 3 portion will enroll 50 additional patients and is expected to commence 12 months after the Phase 2 begins. Patients in this portion of the trial will receive six vaccines as well as an additional booster regimen of ProscaVax. The trial’s endpoints will be reduction in PSA progression and increased immune responses post-vaccination.
Contingent upon clinical results meeting expectations in demonstrating safety and efficacy, OncBioMune intends to seek commercialization of ProscaVax in Mexico through a Preliminary Marketing Authorization. OncBioMune has been advised that this has the potential to happen in as little as 24 months from the commencement of the Phase 2 study.
============================
OvcaVax
BATON ROUGE, La., Oct. 17, 2017 — OncBioMune Pharmaceuticals, Inc. (OTCQB:OBMP) (“OncBioMune” or the “Company”), a revenue-stage biopharmaceutical company engaged in the development of a proprietary cancer vaccine technology, targeted cancer therapies and commercialization of a portfolio of products internationally, is pleased to inform shareholders that the company has begun the process to initiate clinical studies of OvcaVax for ovarian cancer. Built upon the same platform as ProscaVax, the Company’s novel therapeutic vaccine for prostate cancer, OvcaVax is therapeutic ovarian cancer vaccine consisting of a combination of cancer antigen 125 (CA-125), interleukin-2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF).
In the coming weeks, OncBioMune management will be scheduling CA-125 manufacturing under cGMP standards and conducting meetings with contract research organization Theradex Oncology to discuss trial protocol, site location and trial budget. Based upon initial dialogue, the intentions are for a small study enrolling approximately 20 ovarian cancer patients at varying stages of disease to receive OvcaVax in an adjuvant setting following a surgical procedure. Endpoints will include safety, progression-free survival and overall survival.
OncBioMune also intends to pursue select U.S. Food and Drug Administration pathways, such as Orphan Drug designation or Fast Track designation, that can expedite development of OvcaVax and provide extra intellectual property protection should OvcaVax successfully complete clinical trials and be approved for commercialization in the future.
“During our in-house trials, we previously treated four ovarian cancer patients with advanced disease with no signs of toxicity. That data combined with hundreds of other patients receiving vaccinations from the platform technology has us confident in the safety profile of OvcaVax,” commented Dr. Jonathan Head, Chief Executive Officer at OncBioMune. “Recently, we collected very compelling data on ProscaVax eliciting an increased immune response and slowing tumor progression in prostate cancer and believe the same is possible in late-stage ovarian cancer, where women are left with very few therapeutic options. I firmly believe that immunotherapeutic cancer vaccines will one day be a part of standard of care for hard-to-treat disease. Our vaccine technology is on the leading edge of this paradigm shift, so I am eager to initiate these studies of OvcaVax concurrent with the mid-stage studies of ProscaVax.”
Tretinoin
successfully achieved development milestones in formulation and stability with tretinoin, also known as all-trans retinoic acid (ATRA), an oral drug for the treatment of Acute Promyelocytic Leukemia (APL).
OncBioMune owns the commercialization rights for tretinoin throughout Mexico, Central America and Latin America.
http://oncbiomune.com/2017/09/29/oncbiomune-cmo-producing-first-batch-of-tretinoin-for-acute-promyelocytic-leukemia/
========================
OncBioMune To Become Exclusive Distributor of Lipomed Drugs in Mexico
http://oncbiomune.com/2017/08/10/oncbiomune-to-become-exclusive-distributor-of-lipomed-drugs-in-mexico/
OncBioMune Submits Application to Commercialize Anti-Rabies Drug in Mexico
http://oncbiomune.com/2017/08/03/oncbiomune-submits-application-to-commercialize-anti-rabies-drug-in-mexico/
OncBioMune Acquires Propolis Product Line from German Partner
http://oncbiomune.com/2017/07/20/oncbiomune-acquires-propolis-product-line-from-german-partner/
I'll update this more when I have time.
"Perfection is not attainable, but if we chase perfection we can catch
excellence." Vince Lombardi
Do your research! Play the TA. All posts are my opinion.
Recent THER News
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:29:33 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 02/20/2024 08:35:09 PM
- Form 10-K/A - Annual report [Section 13 and 15(d), not S-K Item 405]: [Amend] • Edgar (US Regulatory) • 02/15/2024 10:28:54 PM
- Form NT 10-Q - Notification of inability to timely file Form 10-Q or 10-QSB • Edgar (US Regulatory) • 02/15/2024 08:00:37 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 02/03/2024 01:10:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/26/2024 11:28:46 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/26/2024 11:17:03 PM
- Form NT 10-K - Notification of inability to timely file Form 10-K 405, 10-K, 10-KSB 405, 10-KSB, 10-KT, or 10-KT405 • Edgar (US Regulatory) • 12/29/2023 04:26:15 PM
- Form 3 - Initial statement of beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 09:51:04 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM






