Friday, June 16, 2017 2:11:43 PM
KEVETRIN: PRECLINICAL STUDY OF A NEW COMPOUND IN ACUTE MYELOID LEUKEMIA
Author(s):
Roberta Napolitano
Bioscience Laboratory, IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Serena De Matteis
Bioscience Laboratory, IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Silvia Carloni
Bioscience Laboratory, IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Giorgia Simonetti
Department of Hematology and Oncological Sciences L. and A. Seragnoli, Bologna, Italy
Gerardo Musuraca
Hematology Unit IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Alessandro Lucchesi
Hematology Unit IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Daniele Calistri
Bioscience Laboratory, IRCCS, Istituto Scientifico Romagnolo per lo Studio e la Cura del Tumori (IRST) Medola Italy
Antonio Cuneo
Department of Medical Sciences, University of Ferrara-Arcispedate Sant'Anna, Ferrara, Italy
Krishna Menon
Cellceutix Corp, Beverly, US
Giovanni Martinelli
Department of Hematology and Oncological Sciences L. and A. Seragnoli, Bologna, Italy
Abstract: E904
Type: Eposter Presentation
Background
Acute Myeloid Leukemia (AML) is a heterogeneous disorder defined by clonal expansion of immature myeloid cells that infiltrate bone marrow and other tissues. AML therapeutic strategies remain unchanged since 1970 and the majority of patients often eventually relapse and die due to disease progression. Tumor protein p53 transcription factor is a key regulator of several cellular pathways, such as DNA repair, cell cycle, apoptosis and angiogenesis. It is mutated in 8-14% of AML cases and its mutations are commonly associated with a complex karyotype. Kevetrin is a new molecule compound, proposed by Cellceutix, with the ability to target both wild type and mutant p53 tumors.
Aims
The aim of this project is to explore cellular and molecular alterations induced by Kevetrin, focusing on its role in the p53 pathway.
Methods
Kevetrin was kindly provided by Cellceutix, dissolved and stored at 4°C in sterile water in a 600 µg/ml stock solution, and diluted in medium immediately before use [concentration range in use 15-60 µg/ml]. Cell lines, MOLM-13 and KASUMI-1, were cultured in RPMI 1640 supplemented with 20% heat inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. After 24 and 48 h of treatment MTS, Annexin-V, TUNEL, JC-1 and Active Caspase-3 assays were performed according to manufacturer’s instructions. Proteins were separated by polyacrylamide gel electrophoresis and transferred to 0.2 µm polyvinylidene fluoride membranes. Quantitative analysis was performed with Quantity One software. Statistical analysis was carried out using the paired and unpaired two-tailed Student’s t tests. p values < 0.05 were considered as significant.
Results
Our data indicate that Kevetrin exposure induces cell growth arrest, a great drop of mitochondrial membrane potential and a remarkable increment of Caspase-3 cleaved form, features that contribute to apoptotic cell death in the two cell lines. Cellular changes can be associated with a dose and time-dependent effect in the TP53 mutated cell line (KASUMI-1) but not in the wild type one (MOLM-13), in which we can observe an activity only after 48 h at the higher concentration. Regarding molecular alterations in KASUMI-1 we found a great p53 down-regulation, probably due to Hsp90 reduction, resulting in a less marked formation of the Hsp90-p53 oncogenic complex. We also found a down-regulated p53 active form (Ser15), a reduced expression of p53 targets, p21 and PUMA, and a down-regulation of SIRT-3, that cannot exert its inhibitory activity on p53. The MOLM-13 cell line showed a great p53 reduction, probably related to SIRT-3 up-regulation and Hsp90 down-regulation. Regarding p53 active form, we noticed slight variations in protein expression, suggesting a physiological response of the protein to cellular damage. In accordance with p53 activity, we observed a great up-regulation of p21, probably associated with a drug resistance mechanism; in contrast, PUMA protein was highly down-regulated, suggesting a p53-independent mechanism of action or a feedback regulation of the apoptotic process, after Caspase-3 activation (Figure). In order to better understand drug’s mechanism of action we are performing gene expression profiling after 48 h of treatment with Kevetrin 60 µg/ml.
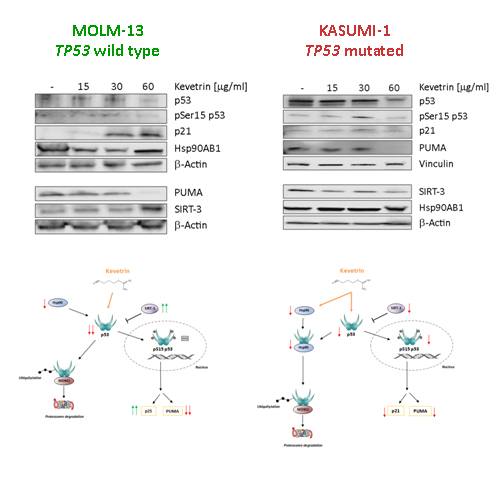
Conclusion
Our results suggest Kevetrin is a promising new drug in AML patients treatment, both in wild type and, even more, in TP53 mutated tumors, through different molecular mechanisms, giving more therapeutic alternatives in the treatment of this disease.
Session topic: 3. Acute myeloid leukemia - Biology
https://learningcenter.ehaweb.org/eha/2017/22nd/180680/roberta.napolitano.kevetrin.preclinical.study.of.a.new.compound.in.acute.html
"Perfection is not attainable, but if we chase perfection we can catch
excellence." Vince Lombardi
Do your research! Play the TA. All posts are my opinion.
Recent IPIX News
- Form 8-K - Current report • Edgar (US Regulatory) • 02/01/2024 01:30:25 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 12/05/2023 09:25:58 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 11/20/2023 09:05:44 PM
- Form NT 10-Q - Notification of inability to timely file Form 10-Q or 10-QSB • Edgar (US Regulatory) • 11/15/2023 01:00:19 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 10/30/2023 08:15:25 PM
- Form 10-K - Annual report [Section 13 and 15(d), not S-K Item 405] • Edgar (US Regulatory) • 09/28/2023 01:00:08 PM
Last Shot Hydration Drink Announced as Official Sponsor of Red River Athletic Conference • EQLB • Jun 20, 2024 2:38 PM
ATWEC Announces Major Acquisition and Lays Out Strategic Growth Plans • ATWT • Jun 20, 2024 7:09 AM
North Bay Resources Announces Composite Assays of 0.53 and 0.44 Troy Ounces per Ton Gold in Trenches B + C at Fran Gold, British Columbia • NBRI • Jun 18, 2024 9:18 AM
VAYK Assembling New Management Team for $64 Billion Domestic Market • VAYK • Jun 18, 2024 9:00 AM
Fifty 1 Labs, Inc Announces Acquisition of Drago Knives, LLC • CAFI • Jun 18, 2024 8:45 AM
Hydromer Announces Attainment of ISO 13485 Certification • HYDI • Jun 17, 2024 9:22 AM









