| Followers | 2543 |
| Posts | 250732 |
| Boards Moderated | 34 |
| Alias Born | 05/06/2014 |
Friday, May 26, 2017 1:37:56 AM
$PMCB Pharmacyte Biotech Inc (OTCMKTS:PMCB) Making A Comeback PMCB

PharmaCyte Biotech, Inc. (OTCMKTS:PMCB), the clinical stage biotechnology company developing medical treatments for cancer and diabetes using the proprietary “Cell-in-a-Box” technology, has recently drawn traders’ attention again. The company is close to an upcoming clinical trial in locally advanced pancreatic cancer. If the company is able to pass this trial, the stock price will become very violent and make an explosive upward move. Some traders are already buying, have a look at the recent upside movements.
Business
PharmaCyte’s technology, Cell-in-a-Box®, is a cellulose-based live cell encapsulation that is used as a platform for the treatment of different types of cancer as well as diabetes. For the treatment of pancreatic cancer, Cell-in-a-Box is used with a combination of low doses of anticancer drug ifosfamide. The combination of both substances is an active “cancer-killing” form. Additionally, the company is also researching the use of its platform for studying the use of cannabinoids for the treatment of cancer. The company believes that this last treatment may help reduce the debilitating side effects usually associated to the cancer treatment. The following picture, found on the website of PMCB, showcases the state of the company’s R&D efforts.
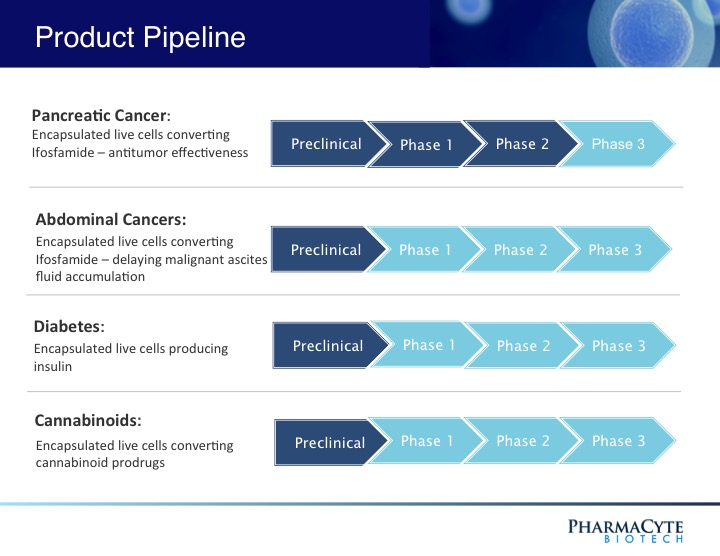
Investors could see from the picture that the most advanced treatment of the company is for the pancreatic cancer. It is involved in the approval of Phase 3 by the FDA, which is the last step before being able to commercialize the product.
Recent Developments
On May 8, 2017, the company released a Q&A article with biotechnology expert Sarah DeMare. Investors can find the press release here. The most important question was made right in the beginning. It was about the list of items that the company has to file in its Investigational New Drug application (IND). The list is quite long. The most important ones are the following:
“(a) Documentation of preclinical work done on the cells.
(b) Toxicology studies.
(c) Documentation of preclinical work done on the capsules themselves.
(d) A wide array of CMC (Chemistry, Manufacturing and Controls) documentation that verifies that the final biologic product (the encapsulated cells) has been produced under current Good Manufacturing Practices (cGMP)-compliant conditions.” Source
In addition, the research disclosed what are the most important points that the FDA will revise, as well as the timing after the process is accepted.
“The FDA is most concerned with patient safety. So, while there may be opportunity to state that certain pieces of information will be available at a later date, those issues that directly impact patient safety are important to include in your IND. From a manufacturing perspective, for a product that is being injected into the body, sterility is of utmost importance to the FDA.” Source
“Following the guidance that the FDA has provided at the pre-IND meeting gives the company the best chance of success at having the IND accepted after the 30-day period.” Source
The timing is quite important for traders as we want to know how long it will take the company take to be able to commercialize the product.
PharmaCyte’s Cannabis Research Program
On April 18, 2017, the company put out another Q&A session with research expert Mark L. Rabe, MD, the Director of PharmaCyte’s Cannabis Program Development. Investors interested can find here the complete interview. We had a look at it and would note the following comments. First of all, we noted that the company did mention that the CBD was useful against cancer.
“Of significance, UNC reported that cannabidiol (CBD), a cannabinoid molecule derived from the Cannabis plant, had anti-cancer effects against several types of cancer cells that were dose-related.” Source
Secondly, Mark L. Rabe noted the size of the market, and the fact that other big companies are also making progress using Cannabis as medicine:
“The current U.S. medical Cannabis industry has been estimated to be worth about $3.0 billion, and it is expected to more than double as more states legalize the use of Cannabis as medicine. There are dozens of companies in the space. Multi-billion-dollar big pharma companies such as Merck, Sanofi-Aventis, AbbVie and Bristol-Meyers Squibb hold cannabinoid-related patents and are conducting cannabinoid-related research.” Source
The investment community is very interested
We went and checked the buzz on the financial forums. We were very surprised with what we found. In total, as of May 16, 2017, there were 1,417 reactions in the Yahoo Finance stock forum. If we take into account the fact that the company does not have a large market capitalization, it means that every movement of the company is extremely studied by the financial community. Thus, if the Phase 3 is accepted, investors should expect a large stock price move.
Solid balance sheet
We were glad to see that the company shows a solid balance sheet. The amount of assets is quite large. Additionally, the amount of cash on the books is remarkable. The only flaw is the amount of intangible assets, which is also elevated.
Period Ending 4/30/2016 4/30/2015 4/30/2014
Current Assets
Cash And Cash Equivalents 1,920.825 2,699.737 3,616.47
Short Term Investments – – –
Net Receivables – – –
Inventory – – –
Other Current Assets 110.026 1,468.281 570.106
Total Current Assets 2,030.851 4,168.018 4,186.576
Long Term Investments 1,572.193 1,572.193 1,572.193
Property Plant and Equipment – – –
Goodwill – – –
Intangible Assets 3,549.427 3,549.427 3,549.427
Accumulated Amortization – – –
Other Assets 7.854 7.854 7.854
Deferred Long Term Asset Charges – – –
Total Assets 7,160.325 9,297.492 9,316.05
Source
Conversely, the amount of liabilities is ten times smaller than the assets and the company has no long term debt:
Current Liabilities
Accounts Payable 487.639 520.366 373.666
Short/Current Long Term Debt – – –
Other Current Liabilities 150 1,000 1,000
Total Current Liabilities 637.639 1,520.366 373.666
Long Term Debt – – –
Other Liabilities – – –
Deferred Long Term Liability Charges – – –
Minority Interest – – –
Negative Goodwill – – –
Total Liabilities 637.639 1,520.366 373.666
Source
Conclusion
The company has very interesting points to note. First of all, the FDA may soon approve the company’s technology for the treatment of Pancreatic Cancer. This is the most important catalyst. Additionally, although the company shows a large amount of shares outstanding, the balance sheet is quite solid and the amount of cash will be sufficient to finance the company’s future operations. To sum up, stay alert, this company may surprise investors in the very near future.
PharmaCyte Biotech, Inc. (OTCMKTS:PMCB), the clinical stage biotechnology company developing medical treatments for cancer and diabetes using the proprietary “Cell-in-a-Box” technology, has recently drawn traders’ attention again. The company is close to an upcoming clinical trial in locally advanced pancreatic cancer. If the company is able to pass this trial, the stock price will become very violent and make an explosive upward move. Some traders are already buying, have a look at the recent upside movements.
Business
PharmaCyte’s technology, Cell-in-a-Box®, is a cellulose-based live cell encapsulation that is used as a platform for the treatment of different types of cancer as well as diabetes. For the treatment of pancreatic cancer, Cell-in-a-Box is used with a combination of low doses of anticancer drug ifosfamide. The combination of both substances is an active “cancer-killing” form. Additionally, the company is also researching the use of its platform for studying the use of cannabinoids for the treatment of cancer. The company believes that this last treatment may help reduce the debilitating side effects usually associated to the cancer treatment. The following picture, found on the website of PMCB, showcases the state of the company’s R&D efforts.

Investors could see from the picture that the most advanced treatment of the company is for the pancreatic cancer. It is involved in the approval of Phase 3 by the FDA, which is the last step before being able to commercialize the product.
Recent Developments
On May 8, 2017, the company released a Q&A article with biotechnology expert Sarah DeMare. Investors can find the press release here. The most important question was made right in the beginning. It was about the list of items that the company has to file in its Investigational New Drug application (IND). The list is quite long. The most important ones are the following:
“(a) Documentation of preclinical work done on the cells.
(b) Toxicology studies.
(c) Documentation of preclinical work done on the capsules themselves.
(d) A wide array of CMC (Chemistry, Manufacturing and Controls) documentation that verifies that the final biologic product (the encapsulated cells) has been produced under current Good Manufacturing Practices (cGMP)-compliant conditions.” Source
In addition, the research disclosed what are the most important points that the FDA will revise, as well as the timing after the process is accepted.
“The FDA is most concerned with patient safety. So, while there may be opportunity to state that certain pieces of information will be available at a later date, those issues that directly impact patient safety are important to include in your IND. From a manufacturing perspective, for a product that is being injected into the body, sterility is of utmost importance to the FDA.” Source
“Following the guidance that the FDA has provided at the pre-IND meeting gives the company the best chance of success at having the IND accepted after the 30-day period.” Source
The timing is quite important for traders as we want to know how long it will take the company take to be able to commercialize the product.
PharmaCyte’s Cannabis Research Program
On April 18, 2017, the company put out another Q&A session with research expert Mark L. Rabe, MD, the Director of PharmaCyte’s Cannabis Program Development. Investors interested can find here the complete interview. We had a look at it and would note the following comments. First of all, we noted that the company did mention that the CBD was useful against cancer.
“Of significance, UNC reported that cannabidiol (CBD), a cannabinoid molecule derived from the Cannabis plant, had anti-cancer effects against several types of cancer cells that were dose-related.” Source
Secondly, Mark L. Rabe noted the size of the market, and the fact that other big companies are also making progress using Cannabis as medicine:
“The current U.S. medical Cannabis industry has been estimated to be worth about $3.0 billion, and it is expected to more than double as more states legalize the use of Cannabis as medicine. There are dozens of companies in the space. Multi-billion-dollar big pharma companies such as Merck, Sanofi-Aventis, AbbVie and Bristol-Meyers Squibb hold cannabinoid-related patents and are conducting cannabinoid-related research.” Source
The investment community is very interested
We went and checked the buzz on the financial forums. We were very surprised with what we found. In total, as of May 16, 2017, there were 1,417 reactions in the Yahoo Finance stock forum. If we take into account the fact that the company does not have a large market capitalization, it means that every movement of the company is extremely studied by the financial community. Thus, if the Phase 3 is accepted, investors should expect a large stock price move.
Solid balance sheet
We were glad to see that the company shows a solid balance sheet. The amount of assets is quite large. Additionally, the amount of cash on the books is remarkable. The only flaw is the amount of intangible assets, which is also elevated.
Period Ending 4/30/2016 4/30/2015 4/30/2014
Current Assets
Cash And Cash Equivalents 1,920.825 2,699.737 3,616.47
Short Term Investments – – –
Net Receivables – – –
Inventory – – –
Other Current Assets 110.026 1,468.281 570.106
Total Current Assets 2,030.851 4,168.018 4,186.576
Long Term Investments 1,572.193 1,572.193 1,572.193
Property Plant and Equipment – – –
Goodwill – – –
Intangible Assets 3,549.427 3,549.427 3,549.427
Accumulated Amortization – – –
Other Assets 7.854 7.854 7.854
Deferred Long Term Asset Charges – – –
Total Assets 7,160.325 9,297.492 9,316.05
Source
Conversely, the amount of liabilities is ten times smaller than the assets and the company has no long term debt:
Current Liabilities
Accounts Payable 487.639 520.366 373.666
Short/Current Long Term Debt – – –
Other Current Liabilities 150 1,000 1,000
Total Current Liabilities 637.639 1,520.366 373.666
Long Term Debt – – –
Other Liabilities – – –
Deferred Long Term Liability Charges – – –
Minority Interest – – –
Negative Goodwill – – –
Total Liabilities 637.639 1,520.366 373.666
Source
Conclusion
The company has very interesting points to note. First of all, the FDA may soon approve the company’s technology for the treatment of Pancreatic Cancer. This is the most important catalyst. Additionally, although the company shows a large amount of shares outstanding, the balance sheet is quite solid and the amount of cash will be sufficient to finance the company’s future operations. To sum up, stay alert, this company may surprise investors in the very near future.
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.










