Thursday, April 13, 2017 10:26:58 PM
Truly a gem gone unnoticed! There’s so much DD that I’ll just add the highlights. Please see the website or OTC page for all the documents…
**Company Expects to Report Initial Revenues in First Half of 2017 and Achieve Profitability by Year-End**
In the first half of 2017, I fully expect we will reach commercialization with one or more of our proprietary drugs. This will enable us to generate the high-margin revenue growth and earnings we have worked so diligently towards for several years.
Lodonal Clinical Trials Completed, New Supplier, Nigerian Drug Approval Won In 2016, we completed the clinical human trials on LodonalTM for HIV in Nigeria, by exceeding primary and secondary endpoints by a substantial margin, and earned approval of its drug regulatory agencies. Lodonal is the first safe, affordable, non-toxic therapy for emerging market patients with compromised immune systems.
To the best of our knowledge, we are the only company that has ever advanced a non-FDA drug, or generic, through an approval process in an emerging market. This is a major accomplishment, one that bodes well for our numerous other initiatives that are similar in nature.
As the largest economy in West Africa, our Lodonal approval in Nigeria allows us to fast track all of the 15 countries in West Africa. Senegal has accepted our New Drug Application for Lodonal. We are seeking approval for three indications. We are on schedule for drug approval in many of the countries that make up the Economic Community of West Africa States (“ECOWAS”) and expect to solidify several of them in 2017.
**2017: Focus on Revenues, Trials, IP and Achieving Profitability**?
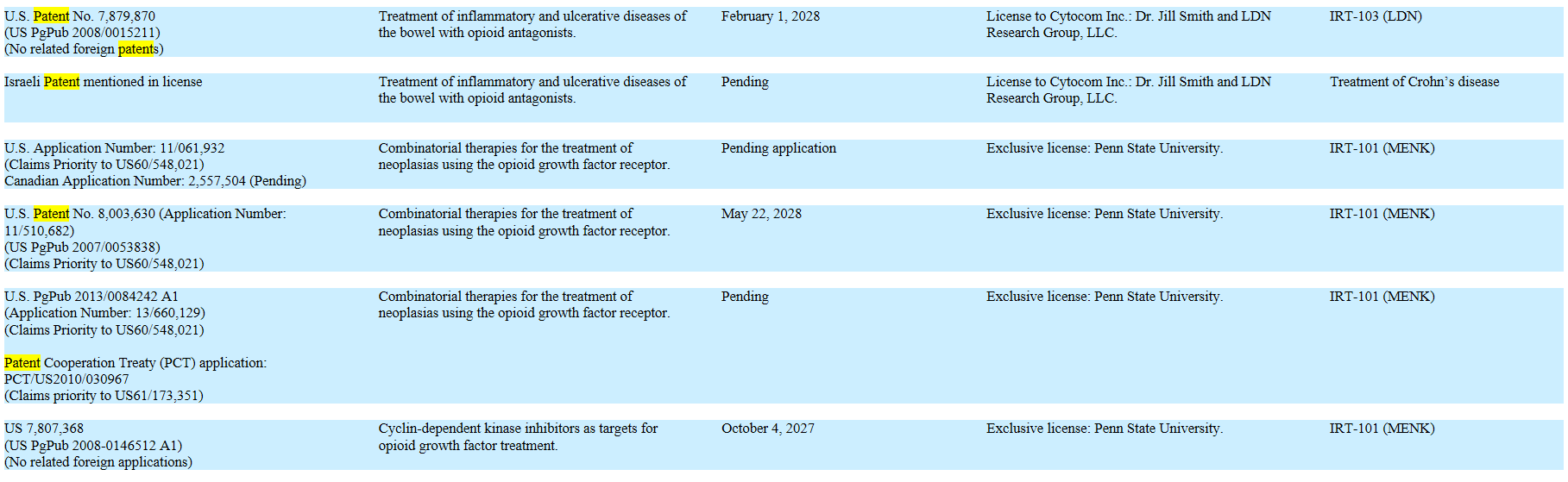
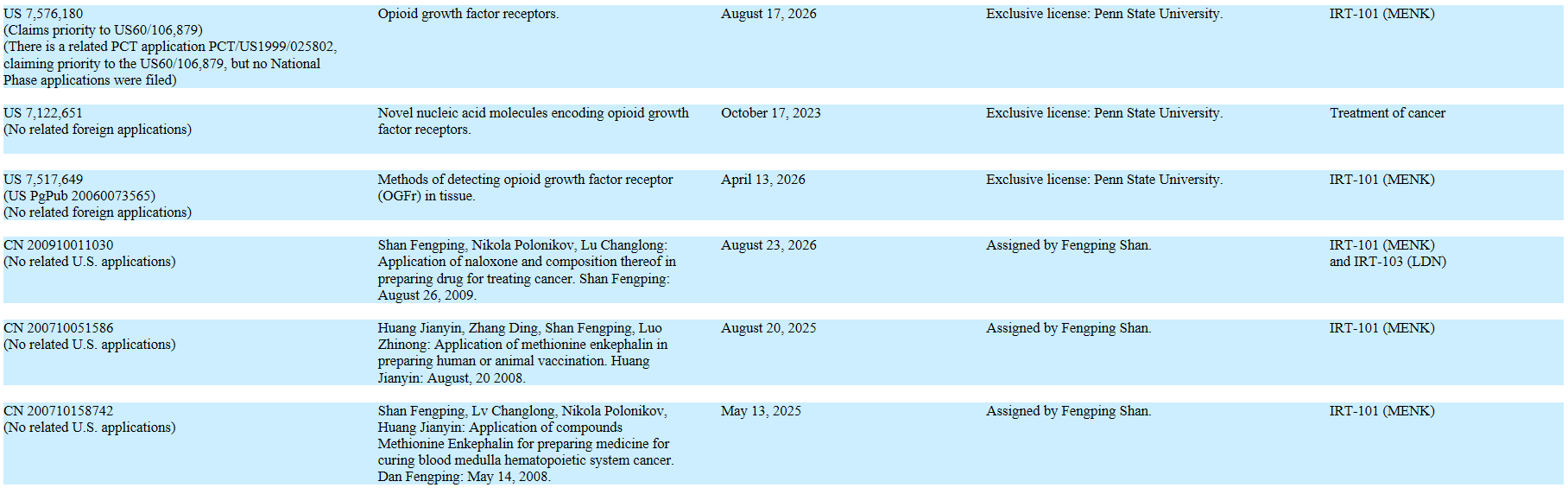
* New IP. In 2016, we filed for three new patents surrounding Lodonal and Met-Enkephalin (“MENK”), and published our research in six medical journals.
? * Revenue and Cash Flow. We expect to commercialize Lodonal in Nigeria, and other West African nations during the first half of 2017. Our internal projections indicate this will generate several million dollars in revenue, at a healthy gross margin, for growing positive cash flow. That cash flow will fund conducting other clinical trials and securing regulatory approvals in other African Nations for additional indications including malaria, opportunistic infections and cancer. Our goal is to be able to fully self-fund our growth by the end of 2017.
? * NAFDAC Marketing Approval. Drug approval does not provide marketing approval, but with the approval of Lodonal by the DR Minister of Health we can now finalize our application for marketing with Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC). The final remaining step is for NAFDAC officers to complete a GMP audit of the facility in Santo Domingo, and this is anticipated by mid-2017. With that, we fully expect to begin distribution and sales in Nigeria with other West African countries to follow.
? * Cytocom. Cytocom Inc. is our affiliate clinical-stage pharmaceutical company focused on advancing IRT-103 (US FDA designator for ‘Lodonal’). It is responsible for the development of our patented therapies with the FDA and EMA. It will lead the US and EMA (“European Medical Association”) clinical trials on IRT-103 including for Crohn’s disease.
? * Advance IMUN’s Growing Drug Pipeline. We have started our second clinical for Lodonal as an adjunct treatment for cancer in Malawi, which is slated for completion in mid-2017, with results to be published in the second half of the year. We also plan a bridging trial with MENK for cancer in Nigeria, capitalizing on our strong relationship with NAFDAC -– amongst other clinical trials being developed or planned.
? * Increase the IP Portfolio. We will continue to protect the value of our clinical research by building intellectual property value as an asset, thus erecting barriers to entry. We filed two new patents surrounding Lodonal in 2016.
? * Establish a Solid Base of Operations in China. Our Chief Science Officer and Vice Director of the Institute of Immunology, China Medical University in Shenyang, China have been treating patients with MENK and our new cocktail therapy throughout 2016 with positive results. Now that our patents are filed for the cocktail, we plan to publish results in 2017 as part of a larger initiative to advance clinical trials, and establish a solid beachhead in this nation of 1.4 billion people as a gateway to Asia. Our goal is to open our cancer clinic in China to treat patients by year-end 2017.
https://www.otcmarkets.com/stock/IMUN/news/UPDATE----Immune-Therapeutics-Issues-Special-Letter-to-Shareholders?id=149724&b=y
Share Structure-
Authorized Shares
500,000,000 a/o Mar 31, 2017
Outstanding Shares
275,640,164 a/o Mar 31, 2017
-Restricted
130,286,587 a/o Mar 31, 2017
-Unrestricted
145,353,577 a/o Mar 31, 2017
Confidence continues to build in our company as, in 2016, our ‘insider’ management and major shareholders continued to add to their IMUN position through both open market purchases and direct investments into the Company.
http://www.immunetherapeutics.com/
And who has the biggest piece of the share pie??? Per the latest 10-K you will see that Mr. Robert J Dailey has 5% ownership in $IMUN. When looking up Robert Dailey and the address listed, it leads you to the company of 843 Lakeshore Partners, LLC. https://www.bizapedia.com/people/california/los-altos/robert-dailey.html But for 843 LP LLC, you will notice another name (William H Crown Trustee from Chicago IL) as it’s partner. Who is this you ask??? ONLY one of FORBES richest families in the early 2000’s! William H Crown owns CC Industries and his net worth is probably less than $1 Billion, but still up there! http://www.chicagobusiness.com/article/20051015/ISSUE02/100024655/crown
The Crown family also runs Henry Crown & Co (Investment firm).
The jewels of Henry Crown and Company shine on like crazy diamonds. Controlled by Chicago's prominent Crown family, Henry Crown and Company is an investment firm that owns or has interests in a variety of business assets. These holdings include stakes in sports teams (the Chicago Bulls and the New York Yankees ), leisure ( Aspen Skiing Company ), banking ( JPMorgan Chase ), and real estate (Rockefeller Center). The company also has a stake in General Dynamics ; after once controlling the company outright, it still has a seat on the board. Affiliate CC Industries holds and manages some of the Crown family's investments.
http://www.hoovers.com/company-information/cs/company-profile.Henry_Crown_and_Company.45498d44f3c35d6b.html
This is important because through an indirect source, the Crown family is funding $IMUN and holds the largest share position in the company through Robert J Dailey!
**IMMUNE THERAPEUTICS STRENGTHENS BALANCE SHEET; Reiterates Outlook for Positive Cash Flow by Mid Year**
https://www.otcmarkets.com/stock/IMUN/news/IMMUNE-THERAPEUTICS-STRENGTHENS-BALANCE-SHEET--nbsp-Reiterates-Outlook-for-Positive-Cash-Flow-by-Mid-Year?id=150868&b=y
What’s the potential impact of selling Lodonal in Africa?
The CEO stated in the first month they project 25,000 patients…and expect to grow to 150,000 patients by the 3rd month. For each 25,000 patients on LDN, it would equate to about $600,000 per month revenue. Those numbers seemed to take into account just the Nigeria selling. But it’s very possible multiple countries could start by EOY 2017 (Kenya, Senegal, etc…) so the impact could be $30+ million in revenues by EOY 2017. 2018 would be significantly higher! THIS IS JUST THE START!
Lodonal is very cheap compared to current drugs that have much harsher effects. This will take a HUGE bit out of the more expensive companies revs. By starting in Africa, $IMUN is paving the way to introduce LDN into the US very soon. This alone would be INSANE since this drug would instantly be much cheaper than any other drug that does similar things with harsher effects out there. Trump will make this transition much easier for $IMUN too…great timing!
Current Patents:


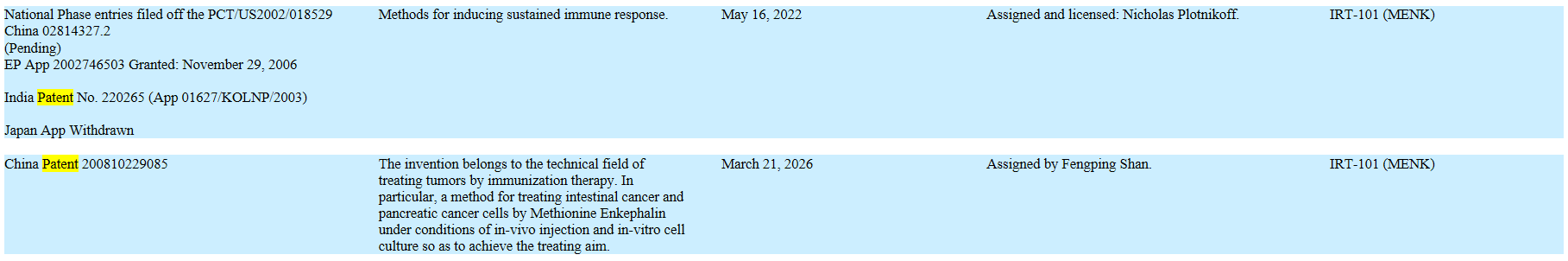
The main thing you need to understand is once this starts selling in Africa, the revs and income will pay for the company needs and dilution will be a thing of the past. It may take a year to be profitable, but this will be monumental for all shareholders! Also, because this drug is safer and so much cheaper than competitors, WHEN they get into the US market it will be game over for the competitors! The potential here is going to be realized very soon and many are missing out on this opportunity to load up on a company that is going to make waves internationally and domestically. $IMUN is in great position to do great things!
There is so much other DD that is on the OTC page under filings that I invite you to look through it all…it’s pretty great! Also go to the iHub message board and read some of ‘rainmakers’ posts. They are very good and informative. It appears there is limited dilution left on this one and that is creating this buying op. Get some while you can!
$$$IMUN$$$
"One of the funny things about the stock market is that every time one person buys, another sells, and both think they are astute." - William Feather.
Avant Technologies Equipping AI-Managed Data Center with High Performance Computing Systems • AVAI • May 10, 2024 8:00 AM
VAYK Discloses Strategic Conversation on Potential Acquisition of $4 Million Home Service Business • VAYK • May 9, 2024 9:00 AM
Bantec's Howco Awarded $4.19 Million Dollar U.S. Department of Defense Contract • BANT • May 8, 2024 10:00 AM
Element79 Gold Corp Successfully Closes Maverick Springs Option Agreement • ELEM • May 8, 2024 9:05 AM
Kona Gold Beverages, Inc. Achieves April Revenues Exceeding $586,000 • KGKG • May 8, 2024 8:30 AM
Epazz plans to spin off Galaxy Batteries Inc. • EPAZ • May 8, 2024 7:05 AM









