| Followers | 603 |
| Posts | 24107 |
| Boards Moderated | 3 |
| Alias Born | 12/06/2009 |
Thursday, March 09, 2017 5:25:50 AM
Here is a compilation of DD that I have put together for new investors in USRM.
US. Stem Cell Inc. Youtube Channel
https://www.youtube.com/user/bioheartinc
US. Stem Cell Inc. Website
http://us-stemcell.com/en/home-2/
US. Stem Cell Inc. Facebook
https://www.facebook.com/USStemCellInc/
US. Stem Cell Clinic Website
http://usstemcellclinic.com/en/home/
Vetbiologics Website
http://www.vetbiologics.com
US. Stem Cell Training Website
https://www.usstemcelltraining.com
_____________________________________________________________
Business Plans:

NEW: ($2,500,000) investment & ten stem cell clinics in the United States
Item 2.01. Acquisition Or Disposition Of Assets.
On March 3, 2017 , the Company entered into an Asset Sale and Lease Agreement with GACP (General American Capital Partners) Stem Cell Bank LLC, a Florida limited liability company (“GACP) to sell to GACP fully depreciated equipment (the “Equipment Assets”) related to the segment of the Company business involving collecting, growing and banking cell cultures (the “Human Banking Business”) for an aggregate of $500,000.
Simultaneous with the sale of the Equipment Assets, the Company leased back the Equipment Assets for a term of three years. The purchase price
for the Human Banking Business was Four Hundred Thousand Dollars ($400,000) for the actual equipment, plus $50,000 for non-equipment assets specifically related to the banking business—plus another $50,000 for customer contracts related to the bank . As consideration for the lease back of the Equipment Assets, the Company will pay base rent of Twenty thousand Dollars ($20,000) per month plus a graduating payment of percentage rent for each of the three years.
In addition, GACP has contractually agreed to invest an additional Two and a half Million Dollars ($2,500,000) to open ten (10) stem cell clinics in the United States within 3 years--with a penalty provision to the benefit of the Company for shortfalls if less than 6 clinics are opened within 24 months.
______________________________________________________________________
Congress passes 21st Century Cures Act, boosting research and easing drug approvals
https://www.washingtonpost.com/news/powerpost/wp/2016/12/07/congress-passes-21st-century-cures-act-boosting-research-and-easing-drug-approvals/?utm_term=.51156066f53f
The Cures Act includes important measures that impact regenerative and stem cell therapies. (Stem cells are unspecialized cells that have the potential to differentiate into specialized cells, and are found in both embryos and adults. As such, they can help to repair the body by dividing to replenish cells that are damaged by disease, injury, or wear.)
Specifically, the act allows for the FDA “to grant accelerated approval for regenerative therapeutic products and directs FDA to consider the unique characteristics of such therapies.” It also requires that the agency “provide a rationale with a determination of whether or not to grant accelerated approval,” according to RAPS. “This section, however, does not change the standards of evidence or limit any other of the authorities of FDA,” the explainer states.
At a mid-September FDA public hearing into the use of human cells, tissues and tissue-based products — in advance of an awaited agency guideline on such use — Brody emphasized the importance of a regulatory pathway that secures patient safety, while also accelerating the review of new regenerative treatments, such as stem cell therapies.
Guess who was at this public hearing with Brody?
Search: Kristin Comella in this document.
https://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/UCM532350.pdf
60 DAYS FDA ACCELERATED APPROVAL!
As described in Section 3033 of the 21st Century Cures Act, a drug is eligible for regenerative advanced therapy designation (RATD) if:
The drug is a regenerative medicine therapy, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, except for those regulated solely under Section 361 of the Public Health Service Act and part 1271 of Title 21, Code of Federal Regulations;
The drug is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and Preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such disease or condition We refer you to the Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics, section III.A., for FDA’s interpretation of whether a disease or condition is serious or life-threatening and whether a drug is intended to treat a serious disease or condition.
The request for RATD must be made either concurrently with submission of an Investigational New Drug application (IND) or as an amendment to an existing IND. We will not grant a RATD if an IND is on hold or is placed on hold during the RATD review.
We generally do not expect you to submit primary data (data sets), but your request for regenerative advanced therapy designation should describe the preliminary clinical evidence. Please include a brief description of any available therapies for the disease or condition, the study design, the population studied, and the endpoint(s) used; and a description of the study results and statistical analyses (e.g., subgroup analyses).
If the RATD request is submitted to your IND as an amendment, the cover letter should specify that the submission contains a REQUEST FOR REGENERATIVE ADVANCED THERAPY DESIGNATION in bold, uppercase letters. If the request is submitted with an initial IND, the cover letter should specify that the submission contains both an INITIAL INVESTIGATIONAL NEW DRUG SUBMISSION and REQUEST FOR REGENERATIVE ADVANCED THERAPY DESIGNATION in bold, upper case letters.
No later than 60 calendar days after receipt of the designation request, the Office of Tissues and Advanced Therapies (OTAT) will notify the sponsor as to whether RATD has been granted. If OTAT determines that the RATD request was incomplete or that the drug development program does not meet the criteria for designation as a regenerative advanced therapy, OTAT will include a written description of the rationale for such determination.
_____________________________________________________________________
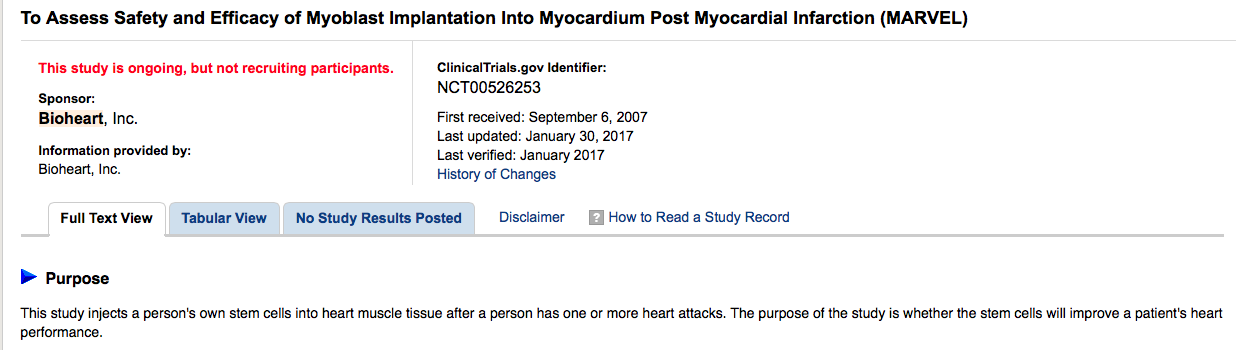
US STEM CELL "THE LEADER" IN PHASE 3
Stem cell therapy for HF is currently being led by the likes of Bioheart (recently renamed US Stem Cell), which has muscle stem cell therapy candidate Myocell in a phase III trial
http://www.pmlive.com/pharma_intelligence/heart_failure_therapy_embarks_on_a_new_era_933222
MYOCELL Clinical Trials Discussed in Video MUST WATCH
Quote from USRM filing:
"We estimate that the products and services we offer through US Stem Cell Training, Vet Biologics, and US Stem Cell Clinics has the potential, although we cannot provide assurances as to if and when it will be accomplished, to drive up to 100 million dollars in cumulative peak annual revenues."
"Our strategy is to expand the revenues generated from each of these operating divisions and to reinvest the profits we generate into our CLINICAL DEVELOPMENT PIPELINE"
Video about MYOCELL And Clinical Trials. MUST WATCH
"A PIVOTAL STUDY " Which means at the end of the study we could commercialize
https://www.youtube.com/watch?v=93_SP8sp194
"WE ARE NOW APPROVED TO FINISH THAT SECOND PART OF THE PHASE 2/3 TRIAL AND IS CONSIDERED AND DESIGNED TO BE A PIVOTAL STUDY"
What is Phase 2/3 ?
Seamless phase 2/3 combination designs (or phase 2/3 designs) hold great promise to bring optimal treatment to patients early and more efficiently. The concept is to combine traditional phase 2 and phase 3 trials seamlessly in operation and inferentially in statistical analysis. As phase 2/3 designs cover a large area of clinical applications, it is not possible to provide comprehensive details for all different clinical applications in this article. Rather, the focus is on providing general descriptions of phase 2/3 designs and their legal foundations under the framework of Title 21 Part 312 of Code of Federal Regulations and Section 355 (b) of the Federal Food, Drug, and Cosmetic Act. This legal framework also provides the basis for correcting misrepresentations of phase 2/3 combination designs found in the literature. Various aspects and underlying principles are discussed in depth with highlights on current difficulties and future challenges to statistical inference.
A pivotal trial is a clinical trial or study intended to provide evidence for a drug marketing approval, e.g. by the United States Food and Drug Administration. Phase III trials are assumed to be pivotal so the phrase is often used for the rare pivotal phase II trials.
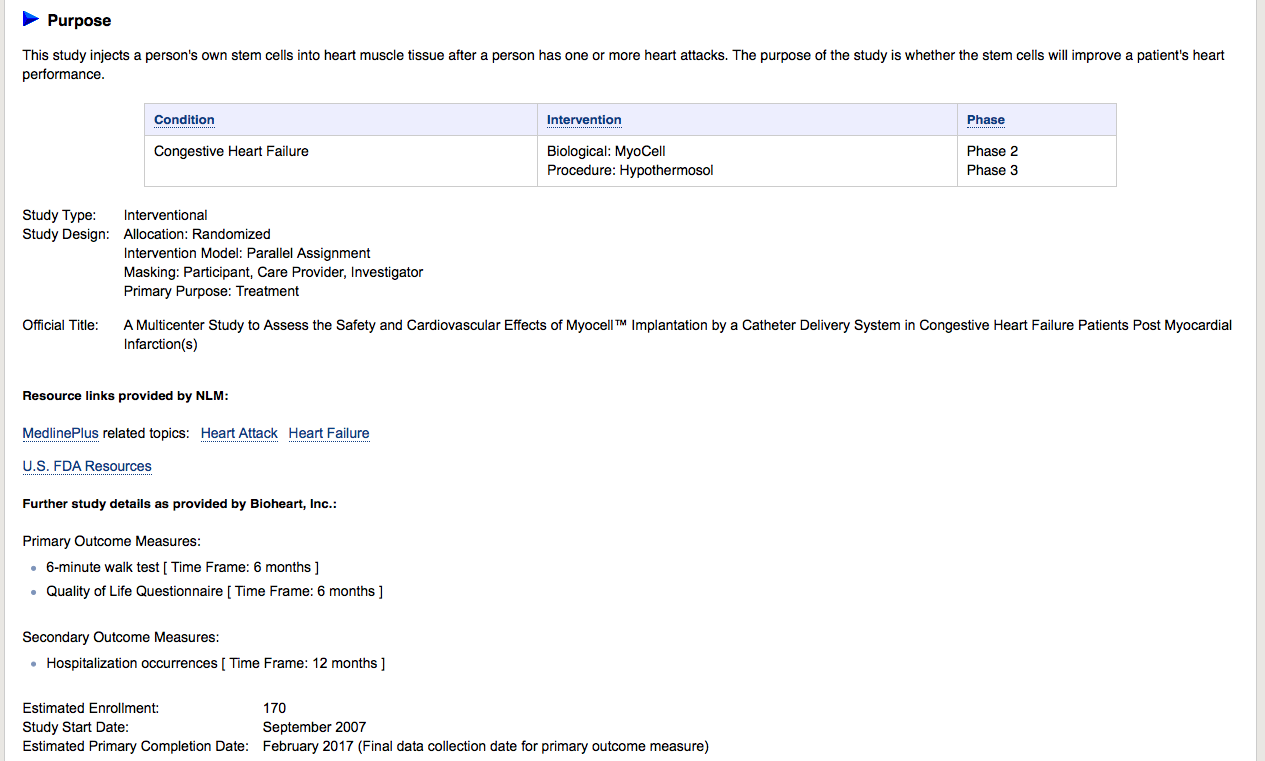
Look what it says on Clinicaltrials.gov:
Estimated Primary Completion Date: February 2017 (Final data collection date for primary outcome measure)
Look at all the Research Related to Myocell:
http://www.zoominfo.com/CachedPage/?archive_id=0&page_id=176407920&page_url=//www.bioheartinc.com/resources.php&page_last_updated=2010-01-07T23:57:31&firstName=Tatsuya&lastName=Shimizu
The latest word from the company is that they will release the results VERY SOON (2-3weeks possibly)
Once these results are released, we may be able to put a much higher value on this company.
https://clinicaltrials.gov/ct2/show/NCT00526253?term=bioheart&rank=3
ClinicalTrials.gov Identifier:
NCT00526253
First received: September 6, 2007
Last updated: January 30, 2017
Last verified: January 2017
We are looking at positive results from most recent data from USRM. According to various sources.
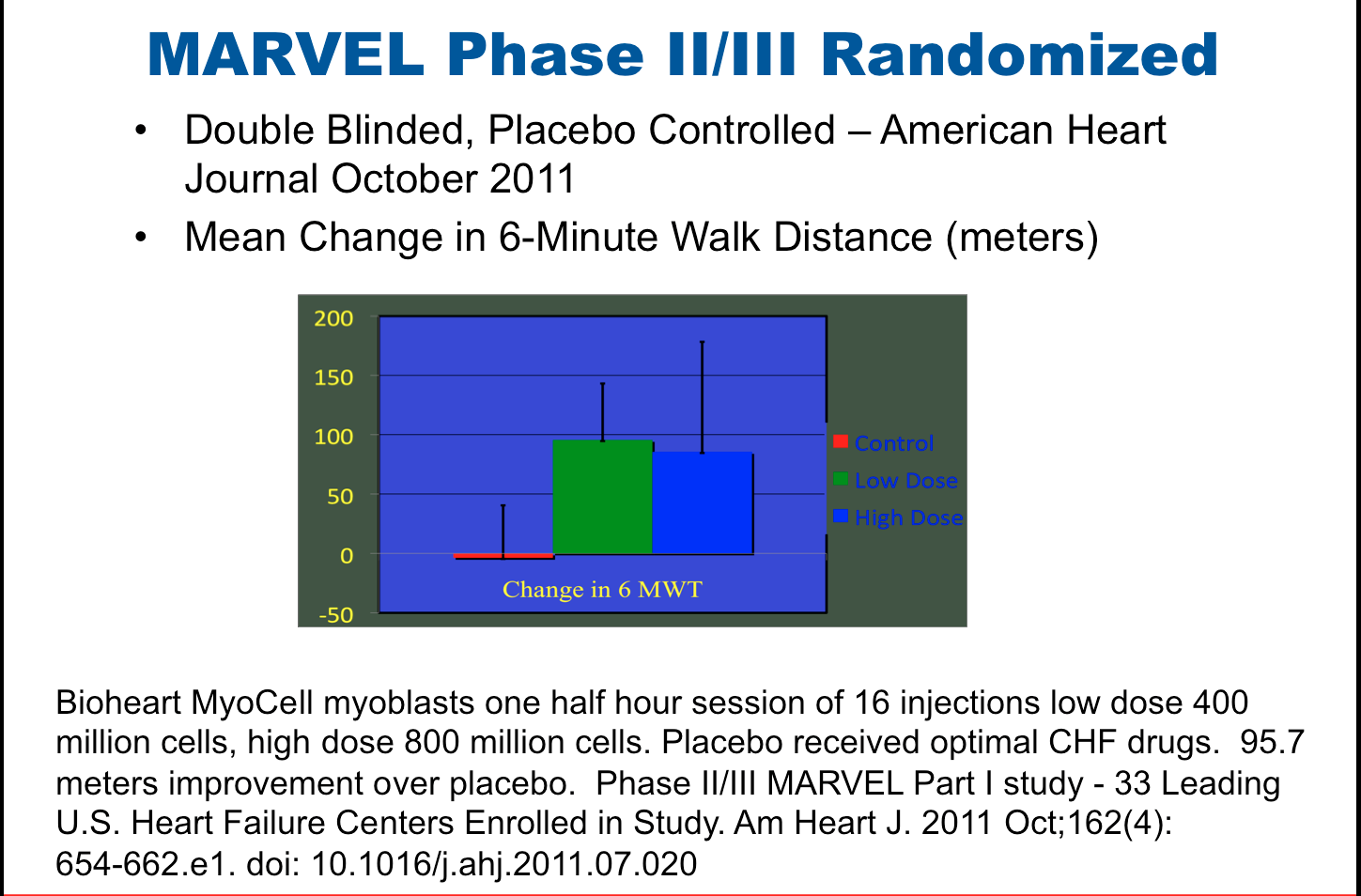

Bioheart's MARVEL Trial Published in the American Heart Journal
"A Double-blind, Randomized, Controlled, Multicenter Study to Assess the Safety and Cardiovascular Effects of Skeletal Myoblast Implantation by Catheter Delivery in Patients with Chronic Heart Failure Following Myocardial Infarction" was published online in the American Heart Journal on September 12, 2011.
MARVEL is the first blinded placebo-controlled trial assessing the safety and efficacy of myoblast's delivered via catheter for patients with advanced symptoms of heart failure due to a heart attack. When compared with placebo, myoblast therapy was associated with sustained (6 months) improvements in six minute walk distance of >90 meters, a clinically meaningful improvement. This means that a congestive heart failure patient may return to a more active lifestyle. Some of the enrolling centers in the trial include: Minneapolis Heart Institute, Florida Hospital Center, The Lindner Center, Jim Moran Heart and Vascular Research Institute, Scripps Green Hospital, and Arizona Heart Institute.


The Marvel Study was lead by who?
Warren Sherman: Cardio3 BioSciences (C3BS) (Euronext Brussels and Paris: CARD), a leader in the discovery and development of regenerative, protective and reconstructive therapies, announces today the appointment of Dr. Warren Sherman as Chief Medical Officer, effective as of 1 November 2014. In this new role, Dr. Sherman will leverage his deep expertise in the cardiovascular and cell therapy fields to support the continued development of Cardio3 BioSciences' product pipeline, both in cell therapies and cardiovascular diseases.
Dr. Christian Homsy, CEO of Cardio3 BioSciences,commented: "We are very pleased to be able to count on Warren Sherman's expertise in clinical application, as well as his renowned scientific outreach for the further development of Cardio3 BioSciences'future projects in regenerative therapies and cardiovascular diseases."
THERE ARE ONLY 3 COMPANIES WITH PHASE 3 HEART FAILURE THERAPIES(that I can find).
1. MESO BLAST
2. CARDIO3
3. US STEM CELL INC.
CHART-2, the Company's second Phase III clinical trial, is intended to assess in the US, the efficacy of C3BS-CQR-1 as a treatment for heart failure of ischemic origin. CHART-2 is designed as a prospective, multi-centre, randomized, sham-controlled, patient-and evaluator-blinded study comparing treatment with C3BS-CQR-1 to a sham treatment. The trial is aimed to recruit a minimum of 240 patients with chronic advanced symptomatic heart failure. The primary endpoint of the trial is the Six Minute Walk Test post-procedure, a commonly used index of cardiovascular performance.
Phase II data published in JACC1 showed C3BS-CQR-1 statistically significant improvement of 77m in six-minute walk distance for the treated patients compared to the control group (p<0.01), which represented a 20% improvement for treated patients versus the control group.
SOUND FAMILIAR? Just like ours imo.
Results of part one of the MARVEL phase II/III study were previously presented by Thomas Povsic, MD, Ph.D., Assistant Professor of Medicine at Duke University. In comparison with the placebo group, myoblast therapy was associated with sustained (6 months) improvements in exercise capacity distance of greater than 90 meters, an increase that would be of great importance to patients if replicated in larger studies.
The study's Principal Investigator Warren Sherman, MD, Director of Stem Cell Research and Regenerative Medicine at the Center for Interventional Vascular Therapy of Columbia University Medical Center stated, "We are gratified that the results of this component of the MARVEL Program will be published in the AHJ, a journal of such high caliber and rigorous peer review process. All involved in the design, conduct and analysis of MARVEL are to be commended, especially those involved at the clinical centers. And special praise is due to Tom Povsic, to his colleagues at Duke and, of course, to our colleagues at Bioheart."
Mike Tomas, President and CEO stated, "The publication of this significant clinical data supporting the use of MyoCell in chronic heart failure patients is an exciting milestone for the company. We remain committed to finalizing part two of the MARVEL trial and eventually commercializing the therapy."
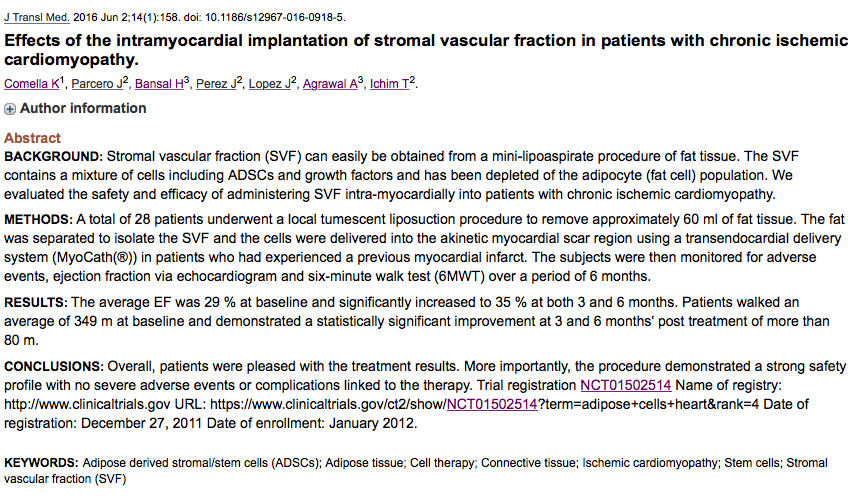
Papers being Published in the Journal of Translational Medicine discussing clinical results..
https://finance.yahoo.com/news/cso-kristin-comella-publishes-paper-173400284.html


______________________________________________________
Clinical Trials ACTIVE:

____________________________________________________________________
USRM (TAM = total addressable market) Worldwide 2016
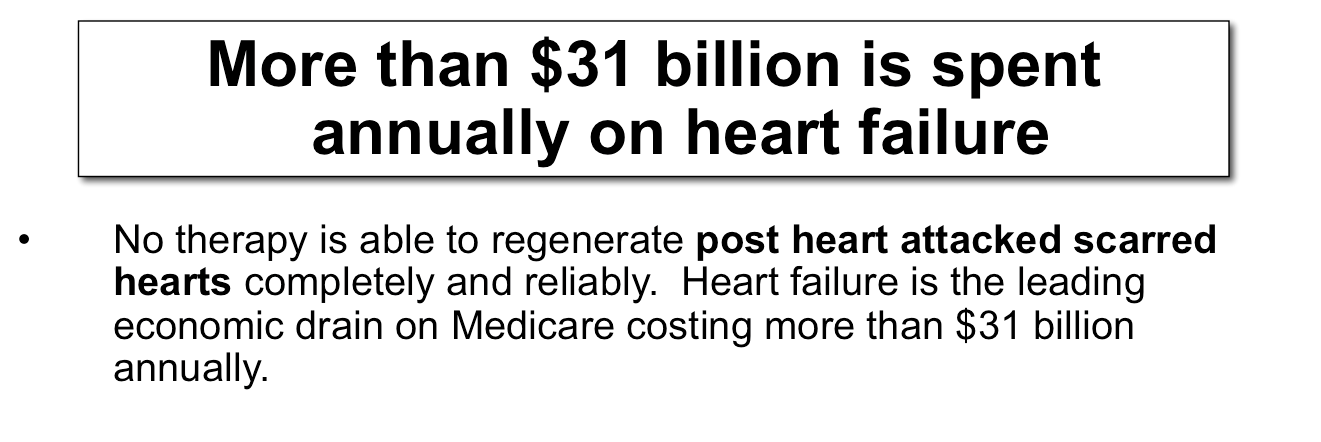
Chronic Heart Failure Treatment:
Degenerative Disc Disease
Spinal Surgery 9.3 Billion
http://www.strategyr.com/PressMCP-1821.asp
spinal and neurostimulation intervention products
$12.6 billion in 2016
https://www.giiresearch.com/report/bc409481-spinal-intervention-markets-surgical-replacement.html
COPD “Chronic Obstructive Pulmonary Disease (COPD)
in 2013 $11.3 billion, and is forecast to reach a value of $15.6 billion by 2019
so we will say 2016 is in the middle at
13.3 Billion for COPD...
_________________________________________________________
USRM HAS MYOCELL ON THE BLUE CROSS BLUE SHEILD INSURANCE WEBSITE!!!
Major insurance company has MyoCell on their website:
https://www.bcbsms.com/index.php?q=member-medical-policy-search.html&action=viewPolicy&path=%2Fpolicy%2Femed%2FAutologous+Progenitor+Cell+Therapy+for+the+Treatment+of+Damaged+Myocardium+due+to+Ischemia.html
_______________________________________________________
Research about Japan and the Stem Cell Revolution, and also links to USRM:
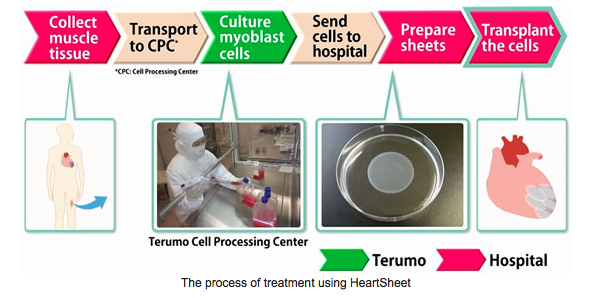
This is Heart Sheet. A "Cellular and Tissue Based Stem Cell Product"
which is a Heart Failure Therapy similar to what US Stem Cell Inc. has with MYOCELL SDF-1.
June 6 ,2016
Terumo Launches HeartSheet®: A Global First Cellular and Tissue-based Product Designed for the Treatment of Heart Failure.
Terumo Corporation (Headquarters: Tokyo, Japan; President and CEO: Yutaro Shintaku) announced today that in late May it commenced sales of HeartSheet - a global first cellular and tissue-based product designed for the treatment of heart failure.
HeartSheet is designed for treating severe heart failure caused by chronic ischemic heart disease. The treatment involves producing skeletal myoblast sheets by culturing skeletal myoblasts contained in muscle tissue that has been taken from the patient’s own thigh, and then transplanting the sheets onto the surface of the patient’s heart.
HeartSheet is a product comprised of two kits: Kit A for collecting the patient’s cells, and Kit B with cultured skeletal myoblasts and tools for producing sheets from cells. Kit A has already been sold to one medical institution, leading to the first case of patient cell collection covered by health insurance. Terumo estimates that the product will be used 20 to 30 cases per year for treatment.
HeartSheet is expected to become a new alternative for the treatment of severe heart failure caused by chronic ischemic heart disease, for which conventional treatments, such as drug therapy and coronary artery bypass surgery,* lack efficacy.
* (artery bypass being..) A surgical procedure for transplanting blood vessels from the legs or other body area to a blocked coronary artery to create an alternative path for blood to flow
In addition, HeartSheet was the first product to be designated as a conditional approval, a system put in place for the purpose of facilitating practical applications of regenerative medicine by the Ministry of Health, Labour and Welfare of Japan. The system is attracting attention among corporations and researchers involved in cellular and tissue-based products, and the utilization of the system is expected to promote advancements in regenerative medicine going forward.
HeartSheet (produced by Terumo) is priced ~$120k. Depending on insurance plans, Japanese patients will still have to pay anything between 5% to 30% of this price
out of pocket.
THATS RIGHT. I SAID IT. 120,000 DOLLARS. Now I am not making this up. Check out the Seeking Alpha Article if you don't believe me...
http://seekingalpha.com/article/3979630-time-invest-stem-cell-biotechs
Japan's "cost calculation method"
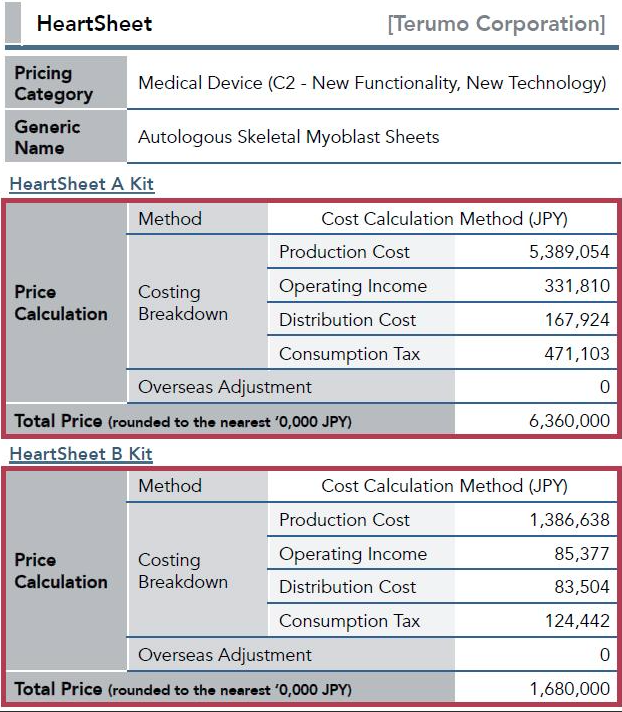
Terumo has been promoting joint research with Osaka University, where clinical research on skeletal myoblast sheets is being carried out by Professor Yoshiki Sawa as part of a project sponsored by the New Energy and Industrial Technology Development Organization (NEDO). Terumo has also participated in a research project on regenerative medicine using cell sheets. It is led by Professor Teruo Okano of Tokyo Women’s Medical University, which is part of a special consortia involved in developing advanced medical care in Japan.
Now... Take A Look at What I found here...
Back in 07' Bioheart signed a letter of intent with ONO Pharmaceuticals... and this wasn't JUST a letter of intent.. read closely... Straight from the USRM filing and various press:
Bioheart Has Opportunity to Commercialize SDF-1 from Ono Pharmaceutical
In 2007, Bioheart signed a Letter of Intent with Ono Pharmaceutical which provided rights to conduct clinical development and testing of SDF-1 to determine the effectiveness of SDF-1 for the treatment of damaged myocardium and tissues following acute myocardial infarction, coronary arterial diseases or heart failure. If the results of this testing are deemed successful, then the parties agree to enter into good faith negotiations in an effort to reach a definitive license agreement that will allow Bioheart to commercialize its SDF-1 product candidate in all territories of the world except Japan.
http://www.businesswire.com/news/home/20100204005998/en/Bioheart-Launches-FDA-Approved-Clinical-Trial-Tests
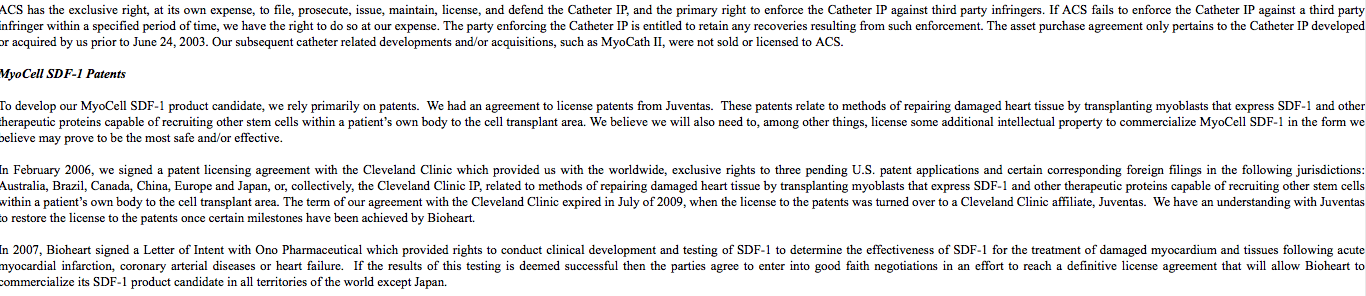
Turns out.. They did do some testing with SDF-1... in 2013
Ono Pharmaceuticals and Yoshiki Sawa... Has Been Testing SDF-1 in MICE in Osaka Japan.

And on top of that.. the results.. looked promising...

Heres another clinical abstract discussing it..
https://www.ncbi.nlm.nih.gov/pubmed/25708182
Y. Sakai was an employee of Ono Pharmaceutical Co. Ltd., and a holder of the patent for ONO-1301 encapsulated in PLGA microspheres (patent numbers WO 2004/032965 and WO 2008/047863).
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0069302
Yoshiki Sawa worked with Yoshiki Sakai on the previous study mentioned with mice...
Dr. Yoshiki Sawa is Professor in the Department of Cardiovascular Surgery, Osaka University Graduate School of Medicine.
Conditions Treated:
Patients with serious heart failure having viable myocardial function who have been declared to have no other available treatment.
Treatment Methods:
(Therapy for Heart Failure Using Autologous Skeletal Myoblast Sheets)
www.medical-excellence-japan.org/en/hospital/020/
Dr. Yoshiki Sawa's name is also found right here from a study done in 2005 relating to myocell: before Bioheart even went public.
http://www.zoominfo.com/CachedPage/?archive_id=0&page_id=1357832886&page_url=//www.bioheartinc.com/article157.php&page_last_updated=2010-01-07T23:57:31&firstName=Tatsuya&lastName=Shimizu

and there is Bioheart's name on the bottom.
And somehow we come up with a former employee of Bioheart:
Employment History:
http://www.zoominfo.com/p/Tatsuya-Shimizu/892982786
And Then Take a Look at all the research related to MYOCELL
Lots of Japanese.
http://www.zoominfo.com/CachedPage/?archive_id=0&page_id=176407920&page_url=//www.bioheartinc.com/resources.php&page_last_updated=2010-01-07T23:57:31&firstName=Tatsuya&lastName=Shimizu
Guys.. I'm not saying it's 100%FOR-SURE BUYOUT situation from Japan, but I'm just saying...
There is a connection between USRM and Japan, Ono Pharmaceuticals and Myocell...
Now.. Back to TERUMO. Ok, so you got TERUMO working with Yoshiki Sawa now...
They want to sell their therapy for 120k AND THEY USE KITS RIGHT?
Take a look at USRM's KITS FOR SALE!!!!!!!!!!
http://files.ctctcdn.com/16692cb3201/f34efd15-aec3-4135-9b73-644636a38ba7.pdf
And this aint OLD NEWS guys.. this is the link that came from Jan 10th,2017 Facebook post:
Ok...So your telling me.. that USRM has FDA approved Clinical Trial in Phase 3 with MYOCELL ?!
Official Title: A Multicenter Study to Assess the Safety and Cardiovascular Effects of Myocell™ Implantation by a Catheter Delivery System in Congestive Heart Failure Patients Post Myocardial Infarction(s)
https://clinicaltrials.gov/ct2/show/NCT00526253?term=bioheart&rank=3
and...
Adipose Derived Cells for Chronic Obstructive Pulmonary Disease
Official Title: An Open
-label, Non-Randomized, Multi-Center Study to Assess the Safety and Effects of Autologous Adipose-Derived Stromal Cells Delivered Intravenously in Patients With Chronic Obstructive Pulmonary Disease
https://clinicaltrials.gov/ct2/show/NCT02041000?term=bioheart&rank=2
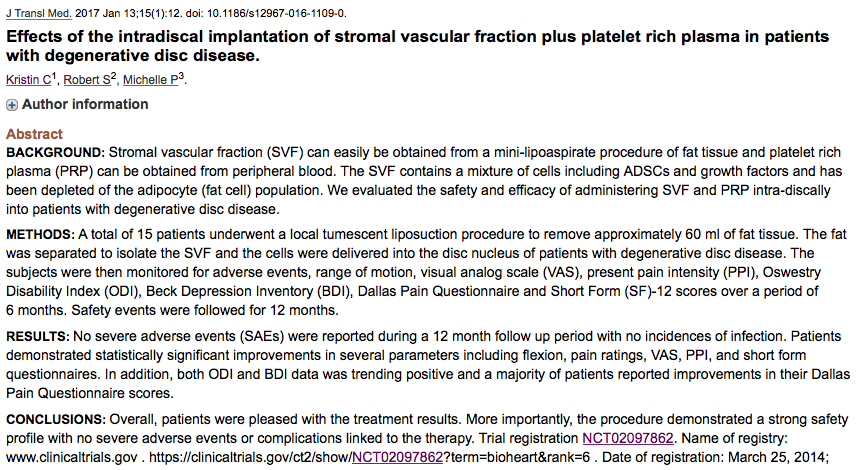
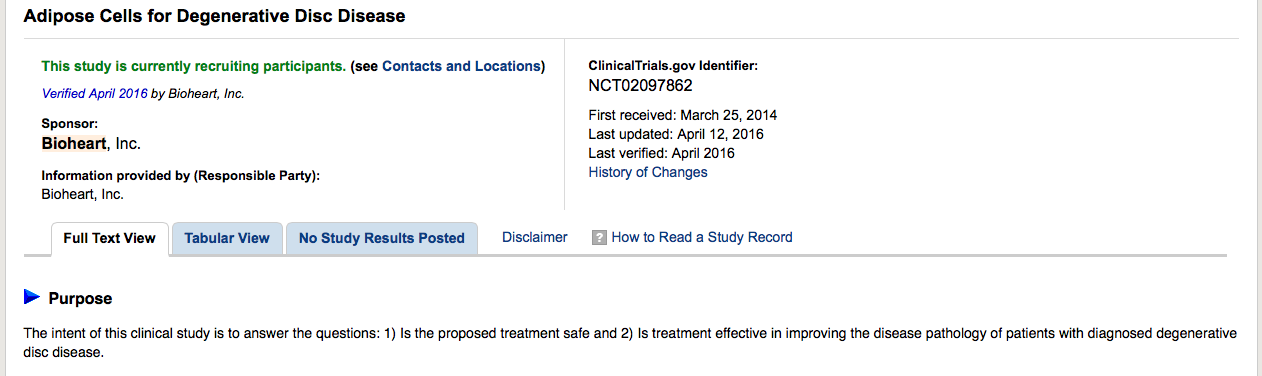
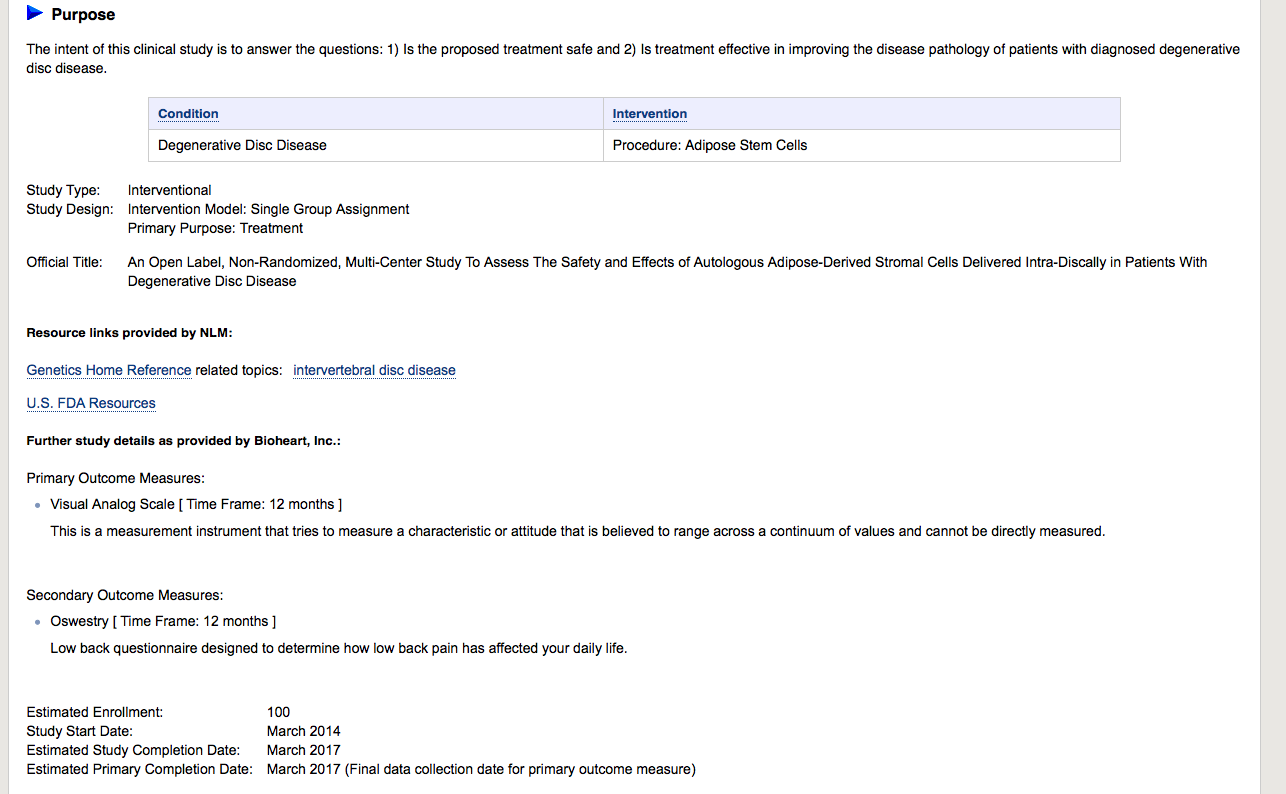
Adipose Cells for Degenerative Disc Disease
Official Title: An Open
Label, Non-Randomized, Multi-Center Study To Assess The Safety and Effects of Autologous Adipose-Derived Stromal Cells Delivered Intra-Discally in Patients With Degenerative Disc Disease
https://clinicaltrials.gov/ct2/show/NCT02097862?term=bioheart&rank=6
and they are selling kits for stem cell collection .. just like... TERUMO, who is SELLING THEIR THERAPY and Kits as a PACKAGE DEAL FOR $120,000 FREAKING DOLLARS?
See from what I can tell.. the difference is the Kits in Japan sold by Terumo, are used in the therapies they are selling because they are ALLOWED to be used in commercial use because the govt of Japan allowed Terumo to commercialize it's therapy..
Right now it looks like USRM is only allowed to sell the kits to other medical practices that want to treat patients in their own clinics for their own trials.. Why you ask.. BECAUSE THE FDA is ALWAYS slowing down progress!
https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm286218.htm
But thats about to change in my opinion. A lot of things are becoming moving parts with the New Administration in place in the US. Govt and National Institute of Health... (Cures Act)
ok so Whats going to happen when and IF USRM starts making commercial sales of these therapies and kits on a worldwide scale? $$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
In the second half of the 20th century, Japan emerged as a world leader in automobiles and consumer electronics. In the first half of this century, the country plans to do the same with stem cells and regenerative medicine.
Japanese government officials and foreign companies working in Japan outlined that vision Wednesday at the annual Stem Cell Meeting on the Mesa. This annual gathering on Torrey Pines Mesa has attracted more than 800 attendees seeking to learn how to bring regenerative therapies to patients -- and make a profit.
With a declining population, more than 25 percent of which are over 65, Japan faces a demographic imperative to extend the healthy lives of its citizens. That vision encompasses repairing damaged organs, replacing worn-out tissues with replacements grown from stem cells, and developing better drugs and biomedical devices.
The United States, with its own aging Baby Boomer population, may see its own future in Japan's struggles to bring advanced biomedical research to patients who need it. And Japan is inviting the U.S. and other nations working in the field to join it, by collaborating with companies and even helping them set up operation in Japan.
Fast track in JAPAN
Under this law, accelerated marketing approval of cell-based regenerative medicine products is possible after demonstration of safety and some signs of efficacy (Phase 1/2 trials). In September of this year, the first two products were approved in Japan under new law.
Clinical trials can be started with just a demonstration of "probable benefit," and products can be approved by successfully conducting a relatively small number of trials, he said.
Already, two cell-based therapies have been approved under the new system, he said. One consists of sheets of muscle cells grown from the patient's own cells, and grafted into the heart to improve function. The other is a treatment of graft vs. host disease, a potentially fatal complication of a bone marrow transplant. That treatment is made from mesenchymal stem cells, which modulate the immune response.
To keep costs down, academic medical centers can outsource cell production to manufacturing centers that can bring economies of scale. And a whole host of well-known Japanese companies such as Nikon, Fujifilm, Panasonic and Olympus are partnering with companies in America and elsewhere.
Predicting a $120-billion Worldwide market, Japan targets stem cells to recharge economy
Japan believes regenerative medicine will grow from a $950-million domestic industry in 2020 to a $10 billion one by 2030, according to a report by Bloomberg News.
And the Japanese expect to tap into a $120-billion global market over the same time span if regenerative medicine fulfills its potential to set off “a medical and industrial revolution.”
...predicts the overall market for stem cells will reach $12bn in 2018, achieving high revenue growth from 2015 to 2025.
"Therapies in myocardial infarction, heart failure and other prominent diseases have reached phase III trials, suggesting the stem cells market will achieve rapid growth during the middle of our forecast period.
http://stemcellfoundation.ca/en/2016/06/29/predicting-120-billion-market-japan-targets-stem-cells-to-recharge-economy/
November 10th, 2015
Ocata Therapeutics of Marlborough, which researches treatments for eye diseases, has agreed to be sold to Astellas Pharma Inc. of Japan for $379 million, the two companies announced late Monday.
Ocata was bought out for 379 Million dollars and they only had a phase 2 trial for blindness (dry age macular degeneration)
(USRM has a the 2nd part of a phase 2/3 trial that is FDA approved!
That means phase 3 is approved for completion!
And With Fast Track Processing, this can be done much faster now!)
https://www.astellas.us/therapeutic/rnd/airm.aspx
(OCATA was once ACTC traded on the OTCBB)
"Positive Stem Cell Clinical OCATA Trial Results"
"Although the primary goal of this small early-stage clinical trial was to assess the safety of the transplanted stem cells, the treatment also had unexpected positive benefits: Ten out of 18 study participants reported improvements in vision, with some subjects reporting dramatic improvements. In addition, the treatment appears to have halted the progression of the disease in 17 of the 18 subjects."
https://www.thestreet.com/story/12914059/1/act-announces-positive-results-from-two-clinical-trials-published-in-the-lancet-using-differentiated-stem-cell-derived-retinal-pigment-epithelium-rpe-cells-for-the-treatment-of-macular-degeneration.html
See guys all it takes is a little bit of positive results...
and then they were approached by BIG PHARMA
Astellas a Japanese pharmaceutical company
Headquarters: Chuo, Tokyo, Japan
Revenue: 11.06 billion USD (2013)
Founded: 2005
Total assets: 14.86 billion USD (2016)
and look what happened...
Astellas Pharma Inc. announced that it has successfully completed, through its indirect wholly-owned subsidiary Laurel Acquisition Inc. (“Laurel”), a tender offer to purchase all issued and outstanding shares of common stock of Ocata Therapeutics, Inc. (NASDAQ
: OCAT) for a price of US$8.50 per share net to the stockholder in cash (“Tender Offer”)
http://www.streetinsider.com/Corporate+News/Astellas+Pharma+Announces+Successful+Completion+of+Ocata+Therapeutics+(OCAT)+Tender+Offer/11299120.html
OCATA is now; Astellas Institute of Regenerative Medicine
https://www.astellas.us/therapeutic/rnd/airm.aspx
Knowing what we know about OCATA, what is the possibility of USRM being approached by the Japanese? After all they have prior dealings with ONO Pharmaceuticals

Positive results with USRM and Myocardial infarction (Heart Attack) could lead to a possible situation where the Japanese would maybe want to be involved with Myocell ...
Bioheart Has Opportunity to Commercialize SDF-1 from Ono Pharmaceutical
In 2007, Bioheart signed a Letter of Intent with Ono Pharmaceutical which provided rights to conduct clinical development and testing of SDF-1 to determine the effectiveness of SDF-1 for the treatment of damaged myocardium and tissues following acute myocardial infarction,
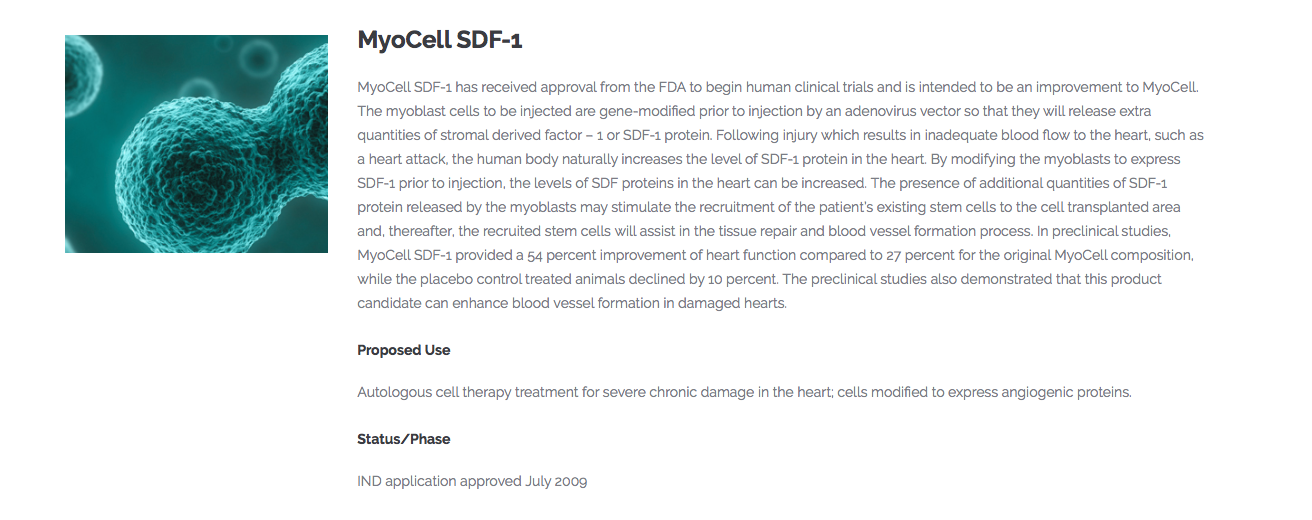
FEB 2017: ONO considers "expansion of development pipeline" and "promotion of global business expansion" as important management tasks at the present time, and also considers M & A (corporate acquisition) to be one of important options
for growth strategy. Regarding M & A, we will determine corporate value and synergy effect in each case. In addition, we regard the scale basically as within the range of cash on hand.
The Average Deal Value for Companies with Heart Disease Treatments: $561 Million
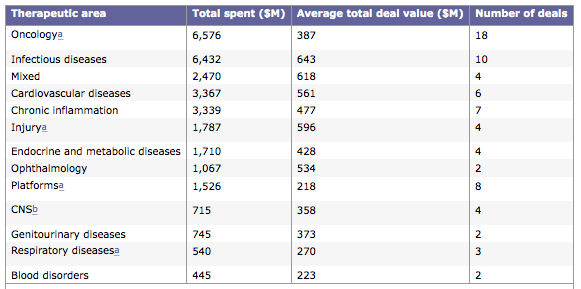
How to Value Biotechs with clinical trials:
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=128849633
Treatment after a Heart Attack:

Japanese Regenerative Medicine Valuations etc..
https://www.mizuhobank.com/fin_info/industry/pdf/mif_141.pdf
Stem Cells Market to USD 25.8 Billion by 2020
http://www.pr.com/press-release/681255
Japan is Breathing Life into the Stem Cell Space, Astellas Pharma NPV (OTCMKTS:ALPMF) & Pluristem Therapeutics Inc. (NASDAQ
:PSTI) Are Benefiting
http://marketexclusive.com/japan-breathing-life-stem-cell-space/9400/
FEBRUARY 2017 "Completion Date"
https://www.premera.com/medicalpolicies/2.02.18.pdf


_______________________________________________________
STEM CELL ACQUISITIONS IN BIG PHARMA :
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=128866450
_____________________________________________________________
Kristen Comella Chief Scientific Officer of USRM
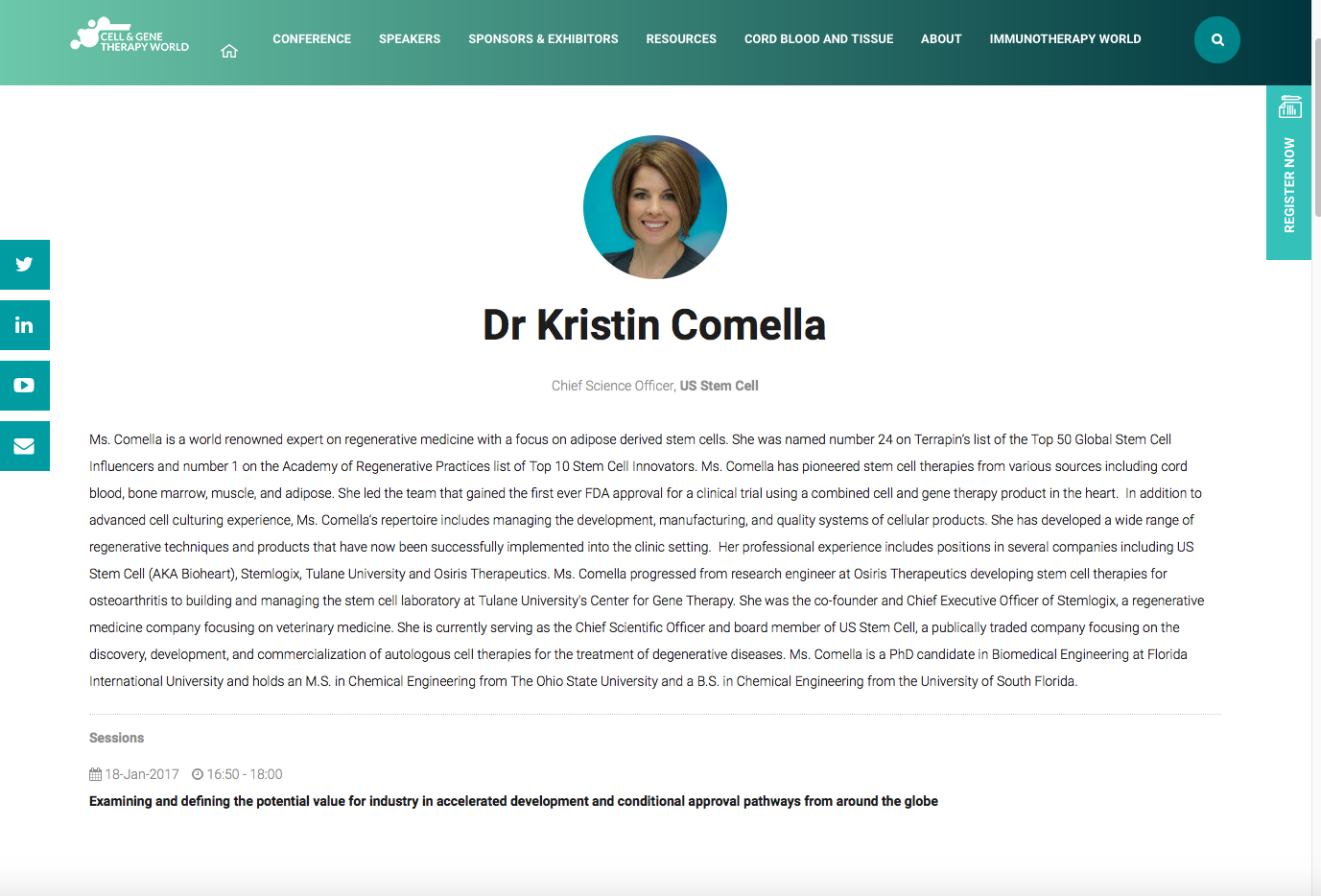

Chief Scientific Officer of US Stem Cell was #24 on the Terrapin’s list of the Top 50 Global Stem Cell Influencers and number 1 on the Academy of Regenerative Practices list of Top 10 Stem Cell Innovators.
Meet Kristen Comella
https://www.youtube.com/watch?v=1sFYmiwbMZM
How Does Stem Cell Therapy Work?
https://www.youtube.com/watch?v=YPaj0RA5u2M
Kristin Comella at RAAD Festival Must Watch!
https://www.youtube.com/watch?v=H4T6WReGPro&t=2s
Kristin Comella | USA | Tissue Science and Regenerative Medicine 2015 | Conferenceseries LLC
https://www.youtube.com/watch?v=SyLNGrjkI8Q
world’s first live stream of a stem cell procedure, featuring our very own Kristin Comella and Michelle Parlo at U.S. Stem Cell Clinic. You can view the recorded livestream at U.S. Stem Cell Clinic’s
http://us-stemcell.com/ceos-blog-october-2016/
USRM's CSO Kristin Comella, will be speaking in London at this conference.
Warren Sherman will be delivering Phase 3 results for CYAD.
also MESO blast will be there discussing Japan and phase 3 for their therapy.
Day 1:
http://www.terrapinn.com/congress/advanced-therapies-regenerative-medicine/Advanced-Therapies-Day-1.stm
Home page, Check out the attendees, major biotech here.
http://www.terrapinn.com/congress/advanced-therapies-regenerative-medicine/index.stm

________________________________________________________________
Warren Buffet Investing in Stem Cells:
His picks include: DaVita (NYSE:DVA), Johnson & Johnson (NYSE:JNJ), and Sanofi (NYSE:SNY).
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=129345290
*TRUM*P supports Stem Cells & cures for rare diseases.
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=129095664
Sarah Hughes a "Guest of Honor" Joint Address to Congress
http://www.khou.com/news/local-woman-to-attend-president-trumps-first-joint-address-to-congress/414545171
____________________________________________________________


___________________________________________________________
Without heart transplantation, a large number of patients with failing hearts worldwide face poor outcomes. By means of cardiomyocyte regeneration, cardiac regeneration therapy is emerging with great promise as a means for restoring loss of cardiac function.

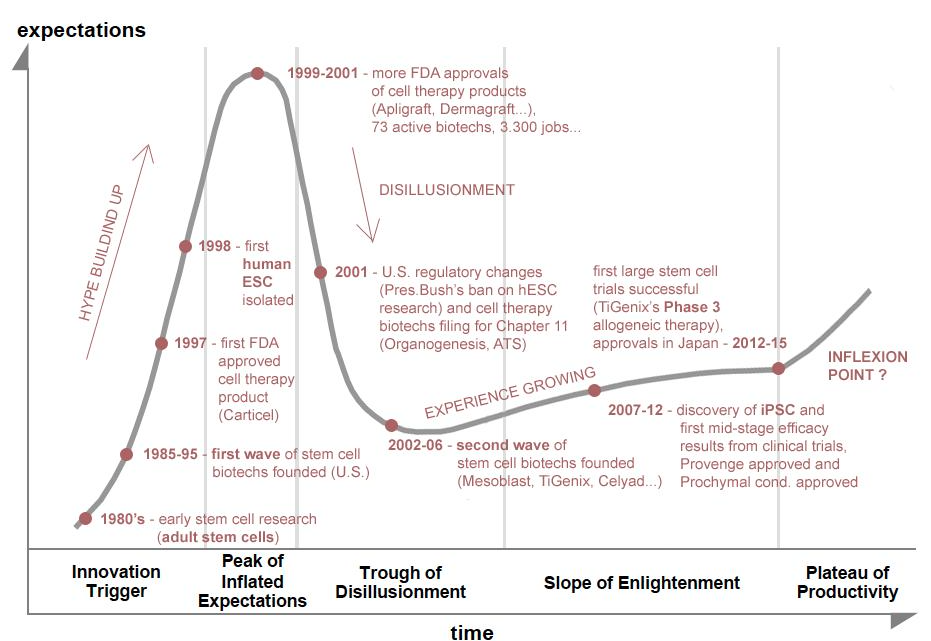
Conclusion: the inflexion point?
The advent of stem cell therapies has been decades in the making. Although developing a new drug typically takes 10 to 15 years from early preclinical work to market approval, early-stage stem cell clinical trials were surrounded by a lot of hype, but unfortunately, convincing efficacy results did not materialize immediately. This had a lasting negative impact on many investors' consideration of the sector as a whole. The technology had to progress further and therapeutic goals had to be adjusted - stem cells were not actually going to cure cancer and heart attacks overnight.
However, as shown in this article, things are really moving on and regenerative therapies are finally getting out of the clinic, offering relevant treatment options
for chronic and acute conditions. While there remain some uncertainties regarding the full commercial transition of cell therapies, the industry is progressing in the right direction and good data is starting to pile up. Besides, the global regulatory environment is becoming less harsh for cell products and the first commercial stem cell therapies have secured very high reimbursement prices in Japan.
So, is it really the right time to invest in stem cell biotechs? While small-cap biotechs generally remain very risky bets, now might be a good time indeed to get to know the field as the more advanced stem cell biotechs appear still cheap despite commercial perspectives that have become very tangible. Several promising products will arrive on the market very soon (Mesoblast's Prochymal in the U.S. in 2018, TiGenix's Cx601 in 2017 in Europe and 2019 in the U.S., etc.) and major Phase 3 results are expected in the coming months, with another string of late-stage data expected in the next 2 years: this might be the inflexion point the stem cell industry has been waiting for...
Stem Cells Are Poised to Change Health and Medicine Forever
By Peter Diamandis - Jan 17, 201716,913
https://singularityhub.com/2017/01/17/stem-cells-are-poised-to-change-health-and-medicine-forever/
Finally, all it might take to light up the sector once again is a first "stem cell blockbuster" - and there are hints that this product might be about to come out of the pipeline.
Could it be that due to circumstances...
USRM is on the Cusp of the INFLEXION POINT in Regenerative Medicine?
From an Ihub Poster:
There is no stopping this train ,I had a major heart attack in 2012 I couldn't walk up a flight of stairs without being out of breath, my cousin told me to go to a hospital in Italy where I had stem cells directly injected into my heart to repair my heart damage, within two weeks I was walking everyday and now I play tennis 3-4 times a week(With a pro).Stem cells is the place to be invested I don't care about anything people have to say. Facts are this procedure works . USRM WILL TRADE @ $1. Repeat ONE DOLLAR very soon. Have a great day! Cause I'm gonna
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=129011796
Kona Gold Beverages, Inc. Announces Strategic Initiatives and Corporate Direction Changes • KGKG • Aug 2, 2024 2:00 PM
POET and Luxshare Tech Expand Product Offerings for Artificial Intelligence Networks • POET • Aug 1, 2024 9:28 AM
Management Discusses Financial Filings of Global Arena Holding Inc., for 10-K 2023 and 10-Q, 1st Quarter 2024 • GAHC • Aug 1, 2024 9:14 AM
VAYK Announces LOI to Acquire $1 Million Home Service Company to Support Airbnb Business • VAYK • Aug 1, 2024 9:00 AM
Duane Forrester Joins INDEXR as SVP of Search • MONI • Jul 31, 2024 11:46 AM
Lingerie Fighting Championships Help Fulfill Death-Bed Promise With First Major Motion Picture • BOTY • Jul 31, 2024 9:00 AM






