Tuesday, December 08, 2015 12:08:55 PM
12-7-15/GEN: “Boosting Upstream & Downstream Output - Sartorius Stedim Biotech Forum Focuses On Next Level Bioprocess Operations”
By: K. John Morrow, PhD (CEO, Newport Biotech)
GEN = Genetic Engineering & Biotechnology News
At the recent Sartorius Stedim Biotech (SSB) Upstream & Downstream Technology Forum in Foster City, CA, (Oct6-7 2015 http://www.sartorius.com/en/upstream-downstream-forum ) speakers dealt with both sides of these technological challenges...
. . .SCALE UP & SCALE DOWN
With tangential flow filtration of protein solutions, researchers are able to concentrate and buffer exchange biotherapeutics to their proper dose and excipients, according to David Briggs, PhD, a research scientist in Mfg. Sciences & Technology at Avid Bioservices. “However, we recognize that at higher protein concentrations this may present a problem, and of course the future of the industry will focus on highly concentrated protein solutions,” he pointed out.
Dr. Briggs discussed his experiences in fine-tuning TFF (Tangential-Flow Filtration), which is also known as cross-flow filtration. The technology is based on running the field stream parallel to the filter device. Small molecules are eliminated, and the larger ones are concentrated in the retentate. While TFF can be employed anywhere in the protein purification process, in general it is only used in the final formulation step.
Dr. Briggs uses diafiltration (DF) in the purification process, by which new buffer is constantly introduced into the flow. With the dilution of the solutes the unwanted molecular species and residual starting buffer components are gradually removed. “During DF, keeping the retentate volume constant is necessary for efficiency and reproducibility,” he told the forum attendees.
A major feature of TFF is that it can be upscaled & downscaled to meet the demands of different protocols without adversely affecting the parameters of the process. The data establish that at large and small scale, flux rates were similar during concentration and that step yields and recovery rates during the entire TFF operation were similar. Finally, at both the manufacturing scale and at lab bench scale, conductivity decreases as a function of diavolumes were similar. . .
http://www.genengnews.com/insight-and-intelligence/boosting-upstream-and-downstream-output/77900575
10-7-15 11:15am Lecture 3
”Successful Scale-Up and Scale-Down of Ultrafiltration Processes in Clinical Manufacturing”
DAVID BRIGGS, PhD, Scientist, MSAT, Avid Bioservices, Inc.
.
.
= = = = = = = = = = = = = = = =
For latest Qtr(7-31-15), Avid ran GP%=51 on Sales of $9.4mm; for last 3 qtrs, GP%=49/GP$=$11.9mm on Sales of $24.1mm. Avid's “committed backlog” = $42mm at 9/2015.
Updated PPHM REVS-BY-QTR TABLE, now thru FY16/Q1 (q/e 7-31-15), per the 7-31-15 10-Q (http://tinyurl.com/pemub47 ) issued 9-9-15.
• Total Revs since May’06: ($138.6mm/Avid + $24.1mm/Govt + $2.4mm/Lic.) = $165.2mm
• Deferred-Revs at 7-31-15, going fwd into FY’16/Q2 (q/e 10-31-15), total $8.3mm, UP from the $6.6mm of Deferred-Revs at 4-30-15 that drove into FY’16/Q1.
• Avid’s Gross-Profit over last 3 qtrs: $12.5mm on revs of $24.4mm (GP% = 49%)
• Recall, Avid Rev$ from Gov’t DTRA Contract work (6/30/08 – 4/15/11, totaling $24.15mm), went into GOVT-REVS, not AVID-REVS, in the Financials.
Avid’s website: http://www.avidbio.com
AVID PROFITABILITY (GROSS*) BY QTR:
QTR Avid-Rev$ CostofMfg$ Gross-Profit$ GP%
FY14Q1 7-31-13 4,581,000 2,670,000 1,911,000 42%
FY14Q2 10-31-13 7,354,000 4,195,000 3,159,000 43%
FY14Q3 1-31-14 3,885,000 2,416,000 1,469,000 38%
FY14Q4 4-30-14 6,474,000 3,829,000 2,645,000 41%
FY14 TOTAL: 22,294,000 13,110,000 9,184,000 41%*
FY15Q1 7-31-14 5,496,000 3,583,000 1,913,000 35%
FY15Q2 10-31-14 6,263,000 4,139,000 2,124,000 34%
FY15Q3 1-31-15 5,677,000 3,113,000 2,564,000 45%
FY15Q4 4-30-15 9,308,000 4,758,000 4,550,000 49%
FY15 TOTAL: 26,744,000 15,393,000 11,151,000 42%*
FY16Q1 7-31-15 9,379,000 4,608,000 4,771,000 51%
*Avid Net-Profit (ie, incl. Selling, G&A) not split out from PPHM-Corp. in the financials.


= = = = = = = = = = = = = = = = = = = = = = =
9-10-15/OutsourcingPharma: “Avid Revs Likely to Grow Substantially”
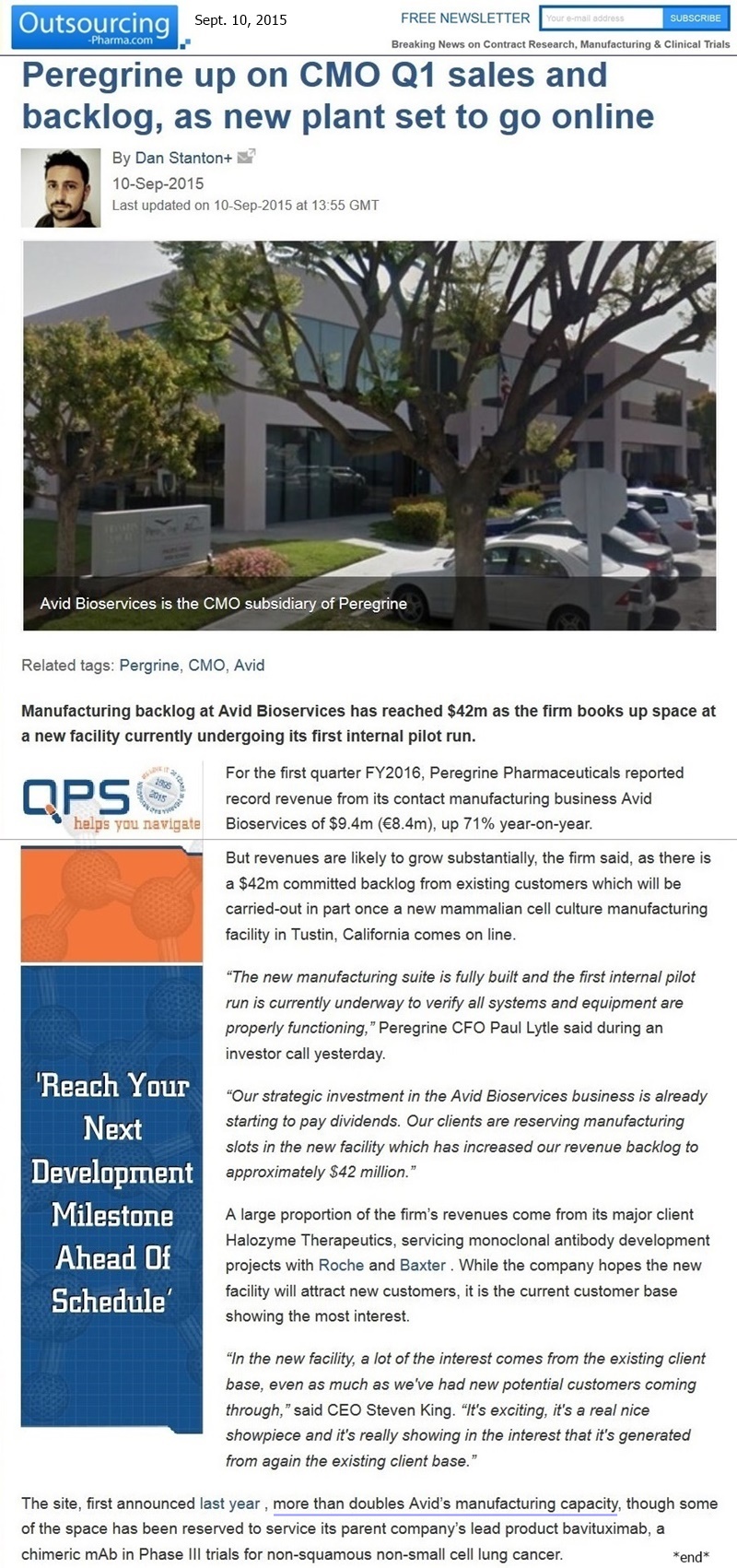
9-10-15: “Peregrine Up on CMO Q1 Sales & Backlog, as New Plant Set to Go Online”
By Dan Stanton, Outsourcing-Pharma
http://www.outsourcing-pharma.com/Contract-Manufacturing/Peregrine-up-on-CMO-sales-and-backlog-as-new-plant-set-to-go-online
Manufacturing backlog at Avid Bioservices has reached $42mm as the firm books up space at a new facility currently undergoing its first internal pilot run. For Q1/FY2016, Peregrine Pharmaceuticals reported record revenue from its contact mfg. business Avid Bioservices of $9.4mm, up 71% year-on-year. But revenues are likely to grow substantially, the firm said, as there is a $42mm committed backlog from existing customers which will be carried-out in part once a new mammalian cell culture mfg. facility in Tustin, CA comes online.
“The new manufacturing suite is fully built and the first internal pilot run is currently underway to verify all systems and equipment are properly functioning,” Peregrine CFO Paul Lytle said during an investor call yesterday. ”Our strategic investment in the Avid Bioservices business is already starting to pay dividends. Our clients are reserving mfg. slots in the new facility which has increased our revenue backlog to approximately $42mm.”
A large proportion of the firm’s revenues come from its major client, Halozyme Therapeutics, servicing monoclonal antibody development projects with Roche & Baxter. While the company hopes the new facility will attract new customers, it is the current customer base showing the most interest.
“In the new facility, a lot of the interest comes from the existing client base, even as much as we've had new potential customers coming through,” said CEO Steven King. “It's exciting, it's a real nice showpiece and it's really showing in the interest that it's generated from again the existing client base.”
The site, first announced last year, more than doubles Avid’s mfg. capacity, though some of the space has been reserved to service its parent company’s lead product bavituximab, a chimeric mAb in Phase III trials for non-squamous NSCLC. END
6-17-15: Avid’s John Haney (ex-Genentech/Pfizer) speaking at BIO-INTL’5/Philly http://tinyurl.com/pnlquu3 & http://tinyurl.com/nl4vbgk
...”Designing & Implementing Avid’s New State-of-the-Art Single-Use Facility for Late Ph.3 & Commercial Prod.” - SK: "We've seen tremendous interest for production in the new facility, both from new & existing clients"
12-10-14: Avid to Double Mfg. Capacity (“expanding client roster; potential commercial launch of bavituximab”) http://tinyurl.com/mmc3qgy & http://tinyurl.com/kmdgq8t
3-24-15: Avid Receives CMO Leadership Awards for Its Commitment to Innovation & Reliability http://tinyurl.com/psep47f
3-12-13: Avid Q3'FY13 GP=$3.3mm; S.King 3-2012, ”We have a profitable CMO, Avid Bioservices" http://tinyurl.com/l97rzm8
Recent CDMO News
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
FEATURED DaBaby and Stunna 4 Vegas's "NO DRIBBLE" Joins Music Licensing, Inc.'s Portfolio • Jun 7, 2024 10:15 AM
Mushrooms Inc. (OTC: MSRM) Announces Significant Share Buy Back by the Board Director and New Strategic Initiatives. • MSRM • Jun 5, 2024 1:32 PM
Hydromer Announces Launch of HydroThrombX Medical Device Coating Technology • HYDI • Jun 5, 2024 10:24 AM
Dr. Michael Dent Finances $1 Million to Drive HealthLynked's Healthcare Transformation • HLYK • Jun 5, 2024 8:00 AM
Avant Technologies Enters Binding LOI to Purchase Dozens of High-Performance, Immersible, AI-Powered Servers • AVAI • Jun 5, 2024 8:00 AM
IQST - iQSTEL Announces $290 Million 2024 Annual Revenue Forecast • IQST • Jun 4, 2024 1:43 PM










