Wednesday, November 04, 2015 10:22:23 AM
Nov4-8 2015: “(SITC) Society for Immunotherapy of Cancer 30th Annual Meeting”, Natl-Harbor MD
”The premier destination for scientific exchange, education, and networking in the Cancer Immunotherapy Field”
SITC = The Society for Immunotherapy of Cancer http://www.sitcancer.org
SITC 2015 Meeting: http://www.sitcancer.org/2015
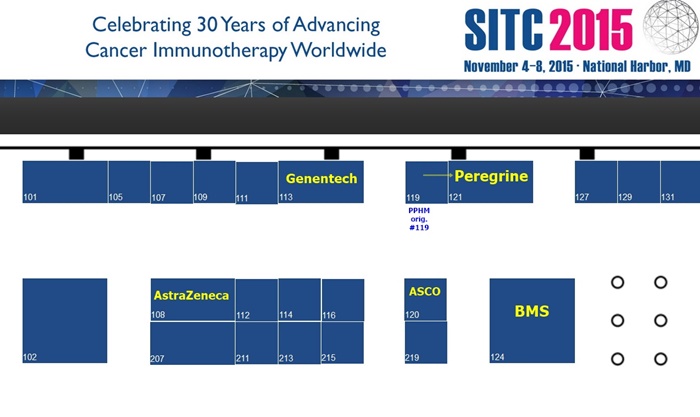
EXHIBITOR: Peregrine Pharm. - booth #121 (directly across from #120/ASCO & BMY/124; AstraZeneca/108 & DNA/113 are close by)
...Floorplan: http://www.eventscribe.com/2015/sitc/exhibitors/index.asp
- - - - - - - -
11-7-15/Sat./12:45-2:00pm Track: “Optimizing Combination Immunotherapy”
Poster: “Targeting Phosphatidylserine Synergizes with Immune Checkpoint Blockade by Inducing De Novo Tumor Specific Immunity”
Presenting Author: Xianming Huang, PhD (UTSW-MC/Dallas)
Xianming Huang 1, Jian Gong 2, Dan Ye 1, Van Nguyen 2, Michael Gray 2, Steven King 2, Jeff Hutchins 2, Rolf Brekken 1, Bruce Freimark 2
...1 UTSW-MC/Dallas
...2 Peregrine Pharmaceuticals, Tustin CA
Direct Link: http://bit.ly/1LS4VYk
= = = = = = = = = = = = = = = = = = = = =
10-15-15: Peregrine & AstraZeneca Expand Collab. w/Ph2/2ndLine-NSCLC Trial, Bavi+durvalumab(MEDI4736), squamous or non-squamous… http://tinyurl.com/q79bkam
10-15-15: AstraZeneca and Peregrine Pharmaceuticals Expand Ongoing Immuno-Oncology Collaboration to Include Phase II Lung Cancer Combination Clinical Trial
Global, Randomized Phase II Trial to Evaluate Immunotherapy Combination of Peregrine's PS-Targeting Bavituximab and AstraZeneca's PD-L1 Inhibitor Durvalumab (MEDI4736) in Previously Treated NSCLC
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=936766
TUSTIN, Oct. 15, 2015: Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM/PPHMP), a biopharmaceutical company focused on developing therapeutics to stimulate the body's immune system to fight cancer, today announced that it has expanded its ongoing cancer immunotherapy clinical collaboration with AstraZeneca to include a second, later-stage trial. The companies will now also evaluate the immunotherapy combination of Peregrine's phosphatidylserine (PS)-targeted immune-activator, bavituximab, and AstraZeneca's anti-PD-L1 immune checkpoint inhibitor, durvalumab (MEDI4736), in a global Phase II study in patients with previously treated squamous or non-squamous non-small cell lung cancer (NSCLC). The randomized Phase II trial will be conducted by Peregrine. . .
ABOUT BAVITUXIMAB: A TARGETED INVESTIGATIONAL IMMUNOTHERAPY
Bavituximab is an investigational chimeric monoclonal antibody that targets phosphatidylserine (PS). Signals from PS inhibit the ability of immune cells to recognize and fight tumors. Bavituximab, the lead compound in Peregrine's immuno-oncology development program, blocks PS to remove this immunosuppressive signal and sends an alternate immune activating signal. Targeting PS with bavituximab has been shown to shift the functions of immune cells in tumors, resulting in robust anti-tumor immune responses.
ABOUT DURVALUMAB (MEDI4736)
Durvalumab is an investigational human monoclonal antibody directed against programmed cell death ligand 1 (PD-L1). Signals from PD-L1 help tumors avoid detection by the immune system. Durvalumab blocks these signals, countering the tumor's immune-evading tactics. Durvalumab is being developed, alongside other immunotherapies, to empower the patient's immune system and attack the cancer.
= = = = = = = = = = = = = = = = = = = = = =
8-24-15: AstraZeneca & Peregrine Collaborate on Bavi+Durvalumab Ph1/1B Trial for “multiple solid tumors”…
Durvalumab=MEDI4736, an anti-PD-L1 immune checkpoint inhibitor.
AZN’s Head/I-O(Robert Iannone): “…Our partnership with Peregrine provides the opportunity to explore an exciting, novel combination that could deliver important clinical benefit to patients across a range of cancers."
8-24-15: AstraZeneca and Peregrine Pharmaceuticals to Collaborate on Immuno-Oncology Combination Clinical Trial
• Collaboration to Focus on Cancer Immunotherapy Combination of Peregrine's PS-Targeting Bavituximab and AstraZeneca's PD-L1 Inhibitor MEDI4736
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=928488
TUSTIN, Aug. 24, 2015: AstraZeneca (NYSE:AZN) and Peregrine Pharmaceuticals, Inc. (NASDAQ:PPHM/PPHMP), a biopharmaceutical company focused on developing therapeutics to stimulate the body's immune system to fight cancer, today announced that they have entered into a cancer immunotherapy clinical trial collaboration. The collaboration will evaluate Peregrine's investigational phosphatidylserine (PS)-signaling pathway inhibitor, bavituximab, in combination with AstraZeneca's investigational anti-PD-L1 immune checkpoint inhibitor, durvalumab (MEDI4736). The planned Phase I/Ib trial will evaluate the safety and efficacy of bavituximab in combination with durvalumab in multiple solid tumors.
Recent CDMO News
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
- Avid Bioservices Reports Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 04/24/2024 09:25:33 PM
- Avid Bioservices Announces Receipt of Deficiency Notice from Nasdaq Regarding Late Form 10-Q • GlobeNewswire Inc. • 03/20/2024 11:00:10 AM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/07/2024 11:30:11 AM
- Avid Bioservices Announces Pricing of Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/07/2024 04:58:48 AM
- Avid Bioservices Announces Proposed Private Placement of Convertible Notes • GlobeNewswire Inc. • 03/06/2024 09:32:07 PM
- Avid Bioservices Announces Certain Preliminary Financial Results for Third Quarter Ended January 31, 2024 • GlobeNewswire Inc. • 03/06/2024 09:31:28 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 03/06/2024 09:30:18 PM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/26/2024 09:57:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/13/2024 12:34:35 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:39:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:38:30 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:37:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:36:27 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/12/2024 12:35:47 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/11/2024 12:56:02 AM
- Form SC 13G/A - Statement of acquisition of beneficial ownership by individuals: [Amend] • Edgar (US Regulatory) • 01/08/2024 09:32:36 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:56:18 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:55:07 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:53:58 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 01/04/2024 12:51:57 AM
- Form SC 13G - Statement of acquisition of beneficial ownership by individuals • Edgar (US Regulatory) • 12/19/2023 09:05:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 12/19/2023 12:34:08 AM
ECGI Holdings Announces LOI to Acquire Pacific Saddlery to Capitalize on $12.72 Billion Market Potential • ECGI • Jun 13, 2024 9:50 AM
Fifty 1 Labs, Inc. Announces Major Strategic Advancements and Shareholder Updates • CAFI • Jun 13, 2024 8:45 AM
Snakes & Lattes Opens Pop-Up Location at The Wellington Market in Toronto: A New Destination for Fun and Games - Thanks 'The Well', PepsiCo, Indie Pale House & All Sponsors & Partners for Their Commitment & Assistance Throughout The Process • FUNN • Jun 13, 2024 8:18 AM
HealthLynked Introduces Innovative Online Medical Record Request Form Using DocuSign • HLYK • Jun 12, 2024 8:00 AM
Ubiquitech Software Corp (OTC:UBQU) Posts $624,585 Quarterly Revenue - Largest Quarter Since 2018 • UBQU • Jun 11, 2024 10:13 AM
Element79 Gold Corp Files for OTCQB Uplisting, Provides Financial Update • ELEM • Jun 11, 2024 9:25 AM










