Friday, August 28, 2015 3:20:01 PM
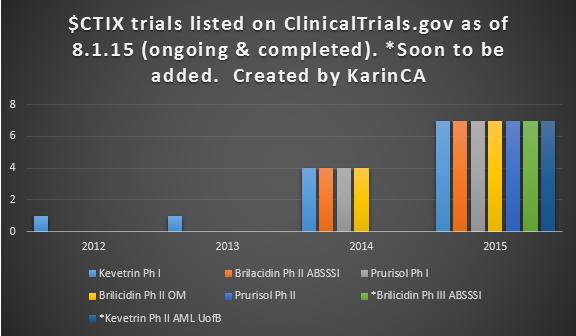
The Phase 2 Clinical Trial: Efficacy and Safety of Prurisol Administered Orally for Active Mild to Moderate Chronic Plaque Psoriasis, is active at 10 locations with 6 having begun recruiting.
(Expanded and updated post with detail on the positive correlation of the mouse model used with clinical observations in humans, and further detail on Prurisol's mechanism. -TOB)
Prior news explained the expedited process that allowed Prurisol to proceed to a Phase 2 Clinical Trial after the Successful Completion of the Bioequivalence and Pharmacokinetic Study of Prurisol and Abacavir Sufate Volunteers: FDA Agrees That Prurisol Phase 2 Study May Begin, with eligibility for the expedited approval pathway.
Prurisol will be eligible for the 505(b)(2) drug approval pathway for the treatment of psoriasis. A potential application to market Prurisol will contain safety and efficacy data from studies conducted by Cellceutix, but at least some of the information required for approval may come from studies not conducted by Cellceutix. In this case, the long term safety data for Ziagen, marketed for over 15 years in the US, will be referenced.
Indeed, the Prurisol Phase 2 Clinical Trial is active and recruiting.
"This Prurisol Phase 2 Clinical Trial is designed to evaluate the efficacy and safety of Prurisol using three different oral daily dose regimens administered to subjects with active mild to moderate chronic plaque psoriasis.
Official Title: A Randomized, Double Blind, Parallel Group, Placebo Controlled Clinical Study of the Efficacy and Safety of Three Different Daily Dosages of Prurisol Administered Orally to Subjects With Active Mild to Moderate Chronic Plaque Psoriasis
The primary efficacy endpoint will be the percentage of subjects with = 2 point improvement in IGA rating as defined by visual inspections of patient lesions [ Time Frame: 84 days]"
Interesting that a $5 billion drug receives only little buyer notice even though the 505(b)(2) pathway might have it on the market by end-2016 or earlier. As noted in the PR, the testing will require no long-term safety data since this data already exists. What this means is the protocol for phase 2 and then 3 can be short-duration study of short-term outcomes.
What do we already know about Prurisol? We know that it CURED psoriasis in xenograft studies. If it works the same in human trials, they will know it is a possible cure within 6 months of the start of the Phase 2 trial. -BigKahuna
Let’s take a look at the validity of the Psoriasis SCID Mouse Model as a predictor of efficacy. (From DD by sox040713)
“The [Prurisol] preclinical studies used the xenografted SCID mouse model. So how well does this model predict efficacy in humans? Etanercept (Enbrel), the psoriasis drug with +$1B in sales, used the same preclinical model.”
Translating clinical activity and gene expression signatures of etanercept and ciclosporin to the psoriasis xenograft SCID mouse model.
Conclusions: Efficacy of etanercept and ciclosporin could be translated to the psoriasis xenograft SCID mouse model.
The following excerpts are from the book: Animal Models of T Cell-Mediated Skin Diseases.
Correlation of the Psoriasis SCID Mouse Model with Clinical Experiences
Published observations on the efficacy of established antipsoriatic treatments in the psoriasis SCID mouse model date back to the year 1999.
The first such publication (Boehncke et al. 1999) adopted a protocol used by the same group for pathogenesis studies. Full-thickness lesional skin was excised from three patients with chronic plaque-stage psoriasis. The skin of each donor was used to derive 12 graphs, each transferred onto a SCID mouse.
[… drugs were tested…]
The results obtained were highly consistent within the treatment groups, but considerable inter-individual variability occurred. Overall, dexamethasone exhibited a robust antipsoriatic efficacy […] This is in line with clinical observations documenting the anti-inflammatory and antipsoriatic efficacy of steroids. Interestingly, the lack of efficacy seen in the case of Bay X 1005 also parallels clinical experience.
[…]
Taken together, the observations published to date document consistency regarding the efficacy of established antipsoriatic drugs in the psoriatic SCID mouse model and clinical practice. This has so far been demonstrated for steroids, dihydroxycholecalciferol, and cyclosporine A. With the exception of clobetasol proprionate, all drugs were given systemically. On the other hand, a compound that failed to show sufficient clinical efficacy to validate further development also showed minimal effects in the psoriasis SCID mouse model. The sole compound where contradicting published data exists is interleukin-10. Still, interleukin-10 is not among those candidates close to be filed for approval as an antipsoriatic treatment. This may indicate that, although principally effective, its potential does not seem to be as good as that of many other biologics.
Extrapolation to the clinical setting is a key requirement for models used in order to identify promising candidates for innovative therapies. This criterion is met by the psoriasis SCID mouse model, which has particular advantages over alternative approaches when it comes to the evaluation of systemic therapies. Besides investing numerous drugs which are already well established in the treatment of psoriasis, the psoriasis SCID mouse model is now widely used to identify candidate compounds suitable for clinical evaluation. Several publications document the application of this model to provide proof of principle for novel therapeutic strategies.
Mechanism of Prurisol: PRINS and IL-20
(See also this important post by ShinSekai researching the Prurisol mechanism. Much more detail and many additional research papers cited. Some content of his research post added to this one.)
Dysregulation of Suppressor of Cytokine Signaling 3 in Keratinocytes Causes Skin Inflammation Mediated by Interleukin-20 Receptor-Related Cytokines
"It has been reported that the cytokines IL-19, IL-20 and IL-24 are highly expressed in the inflammatory sites of psoriasis, and may thus mediate the progression of psoriasis"
Interleukin-20 as a target in psoriasis treatment.
http://www.ncbi.nlm.nih.gov/pubmed/17911452
Abstract
Interleukin-20 (IL-20) is a new member of the IL-10 cytokine family discovered by a structural algorithm. IL-20 transgenic mice displayed skin abnormalities reminiscent of psoriasis, a finding that has prompted the investigation of this new interleukin in relation to this disease. This article reviews the role of IL-20 and its implication in psoriasis. It is shown that IL-20 and its receptors are found in human skin and that IL-20 is involved in proliferation, angiogenesis, and chemotaxis, all characteristics of psoriasis. We demonstrated that IL-20 induced the thickening of human epidermis in vivo; however, this thickening does not seem to be related to a direct effect of IL-20 on hyperproliferation since the growth of normal human epidermal keratinocytes (NHEKs) cultured in vitro was not affected by IL-20. On the other hand, in vitro, IL-20 stimulated human peripheral blood mononuclear cells (PBMCs) to produce proinflammatory cytokines and, in vivo, IL-20 in combination with PBMCs induced psoriasis. This may suggest that IL-20 indirectly exerts its proliferative effects on keratinocytes via immune cells present in the skin. Finally, we found that blocking IL-20 signaling in psoriasis improves psoriasis, suggesting that IL-20 is a potential target in psoriasis treatment.
Polymorphisms in the interleukin-20 gene: relationships to plaque-type psoriasis.
http://www.ncbi.nlm.nih.gov/pubmed/14712309
"Our data indicate that IL-20 gene polymorphisms should have a role in determining susceptibility to plaque-type psoriasis."
"The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS"
"We hypothesize that the deregulation of the PRINS ncRNA may contribute to psoriasis and results in decreased sensitivity to spontaneous keratinocyte apoptosis via the regulation of G1P3."
http://www.ncbi.nlm.nih.gov/pubmed/20377629
One of the most surprising things about this compound is that it is very similar to Lanvis [tioguanine], yet lacks many of the adverse affects typically associated with this cancer drug -often used for psoriasis." –ShinSekai
http://en.wikipedia.org/wiki/Tioguanine
Clinical clearing of psoriasis by 6-thioguanine correlates with cutaneous T-cell depletion via apoptosis: evidence for selective effects on activated T lymphocytes.
RESULTS
After 6 months of treatment, disease severity in 18 of 20 patients showed a significant response to 6-thioguanine: 12 patients were completely cleared of trace disease; 6 showed marked clinical improvement; and 2 did not respond. Patients showed reductions in peripheral blood lymphocytes and total leukocytes, but therapeutic response correlated best with cutaneous T-cell depletion. In vitro assays established that 6-thioguanine has major cytotoxic effects (apparently S-phase specific) on activated T lymphocytes via the induction of apoptosis. Keratinocytes and unactivated T cells, on the other hand, were largely unaffected by incubation with 6-thioguanine.
CONCLUSIONS:
6-Thioguanine is effective for the treatment of moderate to severe plaque-type psoriasis, and may be safe when given for defined periods and with careful hematologic monitoring. The mechanism of action of this drug seems to be the induction of apoptosis in activated T lymphocytes
As a plus, it shares some antimetabolite traits with Methotrexate. We kinda know who the winner is already though ;) –ShinSekai
http://en.wikipedia.org/wiki/Methotrexate
For those of you who want to get a closer look at G1P3 AKA IFI6
http://www.wikigenes.org/e/gene/e/2537.html
Similar also to Cladribine.
Complete remission of chronic plaque psoriasis and gastric marginal zone B-cell lymphoma of MALT type after treatment with 2-chlorodeoxyadenosine. [Cladribine]
Department of Dermatology, University of Vienna, ...
Source: PubMed
ABSTRACT A 58-year-old woman with a 15-year history of chronic plaque psoriasis was diagnosed with gastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type. Topical treatment and ultraviolet radiation for therapy of psoriasis had been of limited efficacy. The patient received intravenous treatment with 0.12 mg/kg per day of 2-chlorodeoxyadenosine (2-CDA) [Cladribine] over 5 days for management of MALT lymphoma, as it was resistant to eradication of Helicobacter pylori. A total of six cycles were administered from June 1999 until November 1999 (cumulative dose 244.8 mg 2-CDA). After 2 months of 2-CDA administration, psoriatic skin lesions improved significantly, and after 3 months, complete remission of skin lesions was observed. Ongoing complete remission of the MALT lymphoma could be achieved after six cycles of 2-CDA administration. After a follow-up period of 34 months, no recurrence of psoriatic lesions has occurred. The patient is at present free of psoriatic plaques and gastric MALT lymphoma. The course of disease in our patient provides evidence for sustained therapeutic efficacy of 2-CDA in chronic plaque psoriasis in the absence of severe side effects except asymptomatic lymphopenia.
http://www.researchgate.net/publication/11014669_Complete_remission_of_chronic_plaque_psoriasis_and_gastric_marginal_zone_B-cell_lymphoma_of_MALT_type_after_treatment_with_2-chlorodeoxyadenosine
Remission of psoriasis after treatment with interferon-alfa and 2-chlordeoxyadenosine [Cladribine] for hairy cell leukemia.
Abstract
2-Chlordeoxyadenosine (2-CdA) [Cladribine] is an antineoplastic/immunosuppressive agent used to treat hairy cell leukemia (HCL), chronic lymphocytic leukemia, and low-grade lymphomas. Its immunomodulatory properties, however, may allow its future use in the treatment of psoriasis. We report a patient with psoriasis and HCL who was treated for 1 week with continuous intravenous infusion of 2-CdA for recurring HCL. Both the psoriasis and the HCL cleared. Four years after 2-CdA treatment, the patient has psoriasis on only 1% of his body surface area. 2-CdA induces lymphocytopenia, which may explain the improvement in this patient's psoriasis.
http://www.ncbi.nlm.nih.gov/pubmed/10426918
Prurisol compared with approved Psoriasis drug Methotrexate
Methotrexate
63% reduction in lesion appearance
48% reduction in serum PRINS
46% reduction in serum IL-20
Lesions reoccurred in 61 days
Prurisol:
84% reduction in lesion appearance
96% reduction in serum PRINS
87% reduction in serum IL-20
No reoccurence of lesions within 180 days
- Link
I'll just say our drug [Prurisol] acts on PRINS and Il-6 related pathways similar to existing drugs just further downstream.
- Link
Seeing as how we see PRINS reduction and Il-20 down regulation, Prurisol appears to be taking on this multifactorial hyper proliferative disease in multiple ways.
Just to add, G1p3 induces the hyper proliferation you see with Psoriasis lesions and they are able to bypass apoptosis. While the down regulation of IL-20 reduces inflammation - ShinSekai
“Prurisol™ is Cellceutix’s anti-psoriasis drug candidate. It is a small molecule (MW=344) that acts through immune modulation and PRINS reduction. Prurisol has been found to be effective against psoriasis in animal models, both in induced psoriasis as well as a xenograft model with human psoriatic tissue. – -Link
We are advancing Prurisol™ for the treatment of psoriasis and recently completed a Phase 1 crossover study to show that Prurisol™ converts to abacavir in humans as it did in animal models. Psoriasis is an immune-mediated inflammatory condition that affects approximately 3 percent of the world’s population, yet despite many available drugs, many people with psoriasis still face a poor quality of life because many approved drugs are still ineffective or come with serious side effects. We believe that a treatment for psoriasis, such as our drug Prurisol, which is taken orally and is a small molecule and not a biologic, is a very desirable drug for development as pills are the preferred method of delivery by patients. We have published images of mouse models demonstrating the robust effect of Prurisol™ in our lab studies.
Based upon the data we have seen to date, we believe the Phase 1 trial met its primary endpoints. The FDA has provided us guidance that a 505(b)(2) pathway is an acceptable approach to expedite the development of Prurisol™. We have submitted our request to meet with the FDA as soon as possible to discuss our protocol to advance Prurisol™ into a larger-scale Phase 2/3 trial.CTIX Update

By SAR, the lead candidate Prurisol is selected. Prurisol in human xenograft model. The top row animals show a clean coat with no evidence of psoriasis, essentially showing that Prurisol cured the psoriasis in the mice. The bottom row shows the untreated control animals.”
PRINS (psoriasis associated RNA induced by stress) is a long non-coding RNA. Its expression is induced by stress, and it may have a protective role in cells exposed to stress. It is over-expressed in the skin of patients with psoriasis. It regulates G1P3, a gene encoding a protein with anti-apoptotic effects in keratinocytes. Overexpression of PRINS may contribute to psoriasis via the down-regulation of G1P3. -PRINS Wiki
Below: A brief overview of clinical and pre-clinical studies of Abacavir for treatment of psoriasis, and studies showing Zidovudin, is effective in the treatment of Psoriasis in Humans with HIV. Abacavir and Zidovudin arenucleoside reverse transcriptase inhibitors.
All below text excerpts, charts and illustrations with links from a variety of sources, including abstracts of studies.
Some random threads below I compiled while looking for a correlation between Abacavir and psoriasis.
Abacavir/lamivudine/zidovudine (INNs, trade name Trizivir) is a pharmaceutical treatment for HIV infection. It is a fixed dose combination of three reverse transcriptase inhibitors patented by GlaxoSmithKline and now marketed by its joint venture with Pfizer, ViiV Healthcare:
Trizivir Wiki
History
In the summer of 1981 the acquired immunodeficiency syndrome (AIDS) was first reported. Two years later the etiological link to AIDS, the human immunodeficiency virus (HIV) was identified. Since the identification of HIV the development of effective antiretroviral drugs and the scientific achievements in HIV research has been enormous. Antiretroviral drugs for the treatment of HIV infections belong to six categories: Nucleoside and nucleotide reverse-transcriptase inhibitors, Non-nucleoside reverse-transcriptase inhibitors, protease inhibitors, entry inhibitors, co-receptor inhibitors and integrase inhibitors. The reverse transcriptase of HIV-1 has been the main foundation for the development of anti-HIV drugs. The first nucleoside reverse-transcriptase inhibitor with in vitro anti-HIV activity was zidovudine. Since zidovudine was approved in 1987, six nucleosides and one nucleotide reverse-transcriptase inhibitor (NRTI) have been approved by FDA. NRTIs approved by the FDA are zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir and emtricitabine and the only nucleotide reverse-transcriptase inhibitor (NtRTI) approved is tenofovir. Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors-wiki

Canadian Guidelines for the Management of Plaque Psoriasis
Antivirals
Primary treatment of HIV with the antiviral drug zidovudine (AZT) can have secondary beneficial effects on skin lesions, including nearly complete or complete clearance of symptoms in up to 90% of patients with HIV-associated psoriasis.54 The same treatment was also beneficial, although less dramatically so, in approximately one-third of HIV-negative psoriasis patients.
Antipsoriatic effects of zidovudine in human immunodeficiency virus-associated psoriasis.
Zidovudine improves psoriasis in human immunodeficiency virus-positive males.
Duvic M1, Crane MM, Conant M, Mahoney SE, Reveille JD, Lehrman SN.
Abstract
BACKGROUND AND DESIGN:
Patients with human immunodeficiency virus (HIV) infection can develop severe psoriasis, which is difficult to treat using conventional therapy. Anecdotal case reports have suggested that administration of zidovudine can improve psoriasis in the HIV-infected patient. An open-label study was conducted to determine the safety and effectiveness of zidovudine therapy in 24 patients with HIV-associated psoriasis and to correlate response with laboratory and clinical variables.
RESULTS:
Of 19 evaluable patients, 90% had either partial (58%) or complete (32%) improvement of their HIV-associated psoriasis during zidovudine therapy. Greater than 75% reduction in the body surface area involved was positively associated with antigenemia and an age younger than 30 years.
CONCLUSIONS:
Zidovudine therapy, at a dosage of 1200 mg/d, appears to be beneficial in the treatment of HIV-associated psoriasis, although long-term relapses occurred and the associated arthritis did not improve.
Zidovudine for the treatment of HIV-negative patients with psoriasis: a pilot study.
Townsend BL1, Cohen PR, Duvic M.
Abstract
BACKGROUND:
Zidovudine, an inhibitor of HIV replication, has been reported to improve psoriasis in HIV-positive patients.
OBJECTIVE:
Our purpose was to evaluate the safety, tolerance, and effectiveness of oral zidovudine for treating psoriasis in HIV-negative patients in a small, open-label study.
METHODS:
Each subject received 200 mg of zidovudine every 4 hours during waking hours, for a total of 1000 mg/day. Treatment was continued for 8 weeks, at which time the patient's response to therapy was evaluated. If a response was evident, treatment was continued for an additional 8 weeks. Clinical response was correlated with histologic changes in skin lesions at 0 and 4 weeks.
RESULTS:
Thirty-three percent of HIV-negative patients with psoriasis showed improvement by up to 80% after 16 weeks of therapy; decreased elevation and scaling of the psoriasis plaques were the most notable changes. No complete remissions occurred.
CONCLUSION:
This is the first report of the use of zidovudine to treat HIV-negative patients with psoriasis. Although zidovudine was well tolerated, it may be more effective in HIV-positive patients with psoriasis.
Psoriasis and HIV Infection
L. Leal,a M. Ribera,a and E. Daudénb Servicio de Dermatología, Hospital Germans Trias i Pujol, Universitat Autònoma de Barcelona, Badalona, Barcelona, Spain Servicio de Dermatología, Hospital Universitario La Princesa, Madrid, Spain
Antiretroviral Treatment
Exacerbation of psoriasis lesions with increasing degrees of immunosuppression suggested antiretroviral treatment as a therapeutic strategy. With the introduction of the first drugs that acted specifically against HIV (with zidovudine [AZT] as the only such drug available), several cases were reported of partial improvement of psoriasis lesions that had previously been refractory to other treatments.(33) The drug was shown to be useful and safe, though its mechanism of action against psoriasis was unknown. It probably acts by reducing keratinocyte proliferation due to its mechanism of interference with DNA synthesis. The introduction of combined therapies has made it very difficult to evaluate the effect of these drugs separately and there is thus nothing in the literature in this regard. As with earlier single-drug therapy, current combined therapies achieve clinical improvement of psoriasis lesions, even in recalcitrant cases resistant to other treatments. (35, 36)
Psoriasis in the Patient With Human Immunodeficiency Virus, Part 2: Review of Treatment
Antiretroviral Therapy
The demonstration of HIV transcripts using in situ hybridization in dermal dendritic cells in psoriatic lesions but not on nonlesional skin suggests that HIV infection might play a direct role in the initiation of psoriatic inflammation. The hypothesis is supported by the observations of an improvement of psoriasis in HIV-infected individuals after antiretroviral t herapy with zidovudine (also known as azidothymidine [AZT]). The apparent clearing of psoriasis with AZT was first reported in 2 HIV positive patients by Duvic et al in 1987 and has been followed by numerous anecdotal confirmations. In an open-label study of 19 assessable HIV-positive patients with psoriasis, 90% had either a partial or complete improvement during therapy with AZT at dosages of 1200 mg daily.
Recent IPIX News
- Form 8-K - Current report • Edgar (US Regulatory) • 02/01/2024 01:30:25 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 12/05/2023 09:25:58 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 11/20/2023 09:05:44 PM
- Form NT 10-Q - Notification of inability to timely file Form 10-Q or 10-QSB • Edgar (US Regulatory) • 11/15/2023 01:00:19 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 10/30/2023 08:15:25 PM
- Form 10-K - Annual report [Section 13 and 15(d), not S-K Item 405] • Edgar (US Regulatory) • 09/28/2023 01:00:08 PM
Branded Legacy Secures Exclusive Extraction Partnership with One of the World's Largest Kava Distributors and Producers • BLEG • May 30, 2024 8:30 AM
ECGI Holdings, Inc. Announces $2 Million Debt-to-Equity Conversion • ECGI • May 30, 2024 8:30 AM
North Bay Resources Reports Assays up to >25% Mg, 0.1% Ni, 0.1% Cu, 0.01% Co, 0.3 ppm Pt at Tulameen Platinum Project, British Columbia • NBRI • May 29, 2024 9:03 AM
One World Products, Inc. Issues Shareholder Update • OWPC • May 29, 2024 8:20 AM
Green Leaf Innovations, Inc. Engages Olayinka Oyebola & Co for Two-Year Audit • GRLF • May 28, 2024 8:30 AM
HealthLynked Introduces AI-Powered Chat Function to Enhance Healthcare Accessibility • HLYK • May 28, 2024 8:00 AM