Friday, May 08, 2015 12:14:23 PM
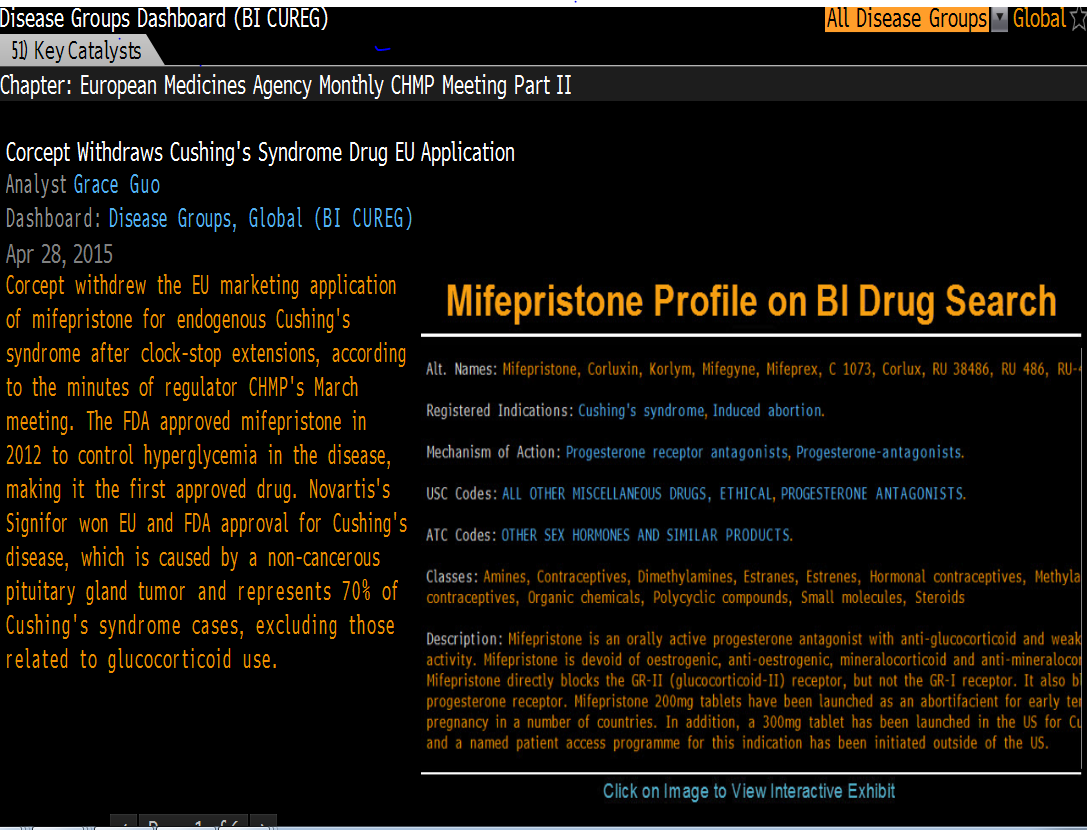
I asked Corcept about the withdrawal and Corcept's CFO, Charles Robb responded. He said, Corcept withdrew Korlym for strategic reasons.
If approved in the EU, Korlym would be tied to the price of Mifepristone when used as an abortifacient — at about 1/4 the price Corcept charges in the USA for Cushing's.
If Corcept's new compound, 125134 were then later approved for Cushing's, 125134's price would be tied to the lower EU price for Korlym.
By withdrawing Korlym, if and when 125134 is approved for Cushing's, there will be less for the EU to use to limit the sales price of 125134.
By withdrawing Korlym, Corcept can fully enroll the trials for 125134 in the EU, without cannibalizing paying patients.
I didn't ask whether or not the withdrawl would affect their revenue guidance for the year but during the conference call, Corcept reaffirmed their earlier guidance $47–53M.
Recent CORT News
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/19/2024 10:34:47 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/06/2024 12:27:19 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/06/2024 12:24:14 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/06/2024 12:08:37 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/06/2024 12:04:11 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 09/05/2024 11:57:22 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 09/03/2024 03:33:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 08/28/2024 09:59:31 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 08/26/2024 07:37:12 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 08/05/2024 11:10:24 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 08/05/2024 10:56:09 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 08/05/2024 10:31:52 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 08/02/2024 08:42:32 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 08/01/2024 05:36:48 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 08/01/2024 03:26:49 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 07/29/2024 08:13:19 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 07/29/2024 08:10:07 PM
- Corcept Therapeutics Announces Second Quarter Financial Results and Provides Corporate Update • GlobeNewswire Inc. • 07/29/2024 08:05:00 PM
- Corcept Therapeutics to Announce Second Quarter Financial Results, Provide Corporate Update and Host Conference Call • GlobeNewswire Inc. • 07/22/2024 08:05:00 PM
- Corcept Announces Presentation of Results From Prevalence Phase of CATALYST Clinical Trial at American Diabetes Association’s Scientific Sessions • GlobeNewswire Inc. • 06/24/2024 08:15:00 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/06/2024 10:21:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/06/2024 01:19:38 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/06/2024 01:15:40 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/06/2024 01:15:01 AM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 06/06/2024 01:13:55 AM
FEATURED Cannabix Technologies and Omega Laboratories Inc. Advance Marijuana Breathalyzer Technology - Dr. Bruce Goldberger to Present at Society of Forensic Toxicologists Conference • Sep 24, 2024 8:50 AM
FEATURED Integrated Ventures, Inc Announces Strategic Partnership For GLP-1 (Semaglutide) Procurement Through MedWell USA, LLC. • Sep 24, 2024 8:45 AM
Avant Technologies Accelerates Creation of AI-Powered Platform to Revolutionize Patient Care • AVAI • Sep 24, 2024 8:00 AM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM






