Wednesday, February 04, 2009 5:49:47 PM
2/3/09 Capacity Results Call/Presentation Summary/Notes
Primary End Point (change in percent predicted Forced Vital Capacity):
Capacity 1 Missed p=0.501. Capacity 2 Hit p=001
. Of note at weeks 12, 24, 36 and 48 both Capacity 1 and 2 had significant P values in FVC (except week 12 Capacity two which was .061)
. Repeat measures analysis over full trial length (retrospective) Capacity 1 p= 0.004, 2 p= 0.001 pulled p < 0.001
Secondary End Points
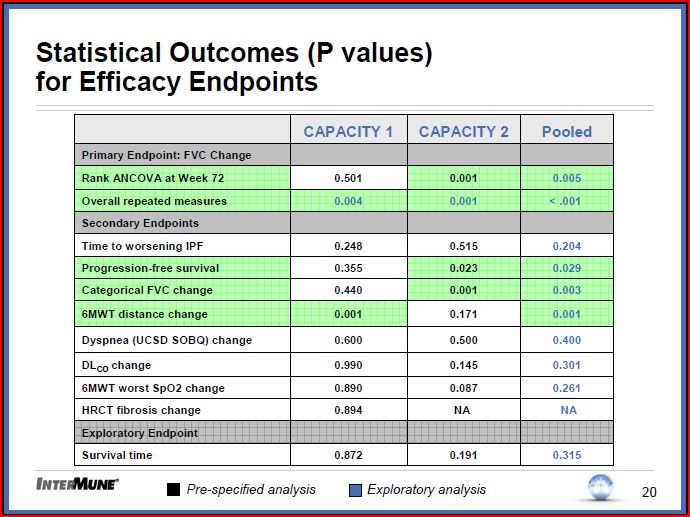
. Company plans to pursue EMEA and FDA filings at this time. When asked if will have dialog with FDA about whether another trial would be needed indicated at this time plan is to proceed with current package.
. General safe and well tolerated AE’s consistent with previous Pirfenidone trials (Rash, Stomach upset)
. No reason given for difference in trial out comes. Mentioned drug discontinuation unlikely to factor and demographics similar in two trials. Some possible areas for further exploration include different sites used for the two trials, placebo group behaving different than historical in Capacity 1 in the later weeks of the trial, and a sicker patient population group in Capacity 1 (specifically Oxygen use 2x that of Capacity 2 and baseline 6MWT about 10% lower than Capacity 2)
Some Speculations (Opinion only do not take as anything else)
1. My guess is it is likely to pass an FDA panel review but I am uncertain on FDA review. I think we get one or the other (FDA or EMEA) to approve with current data perhaps with Phase 4 or label restriction. I don’t know how easy a sale it would be I would push for a Phase 4 Pirfenidone+NAC perhaps 4 arm trial (assuming there is enough preclinical work/safety info to rationalize it). I am not sure if the two are antagonistic, synergistic (or something in between) but I imagine if approved patients would likely be on the combo so it is in the interest of everyone to know. If past trials are an indication and they are somewhat complimentary perhaps it gives a more meaningful result.
2. I put likelyhood of InterMune partnering Pirfenidone in some form as quite high. Keeping in mind that they have several analogs in various stages of preclinical development. I see a package partnership (if not the whole company). I would think a bigger Pharma or company with a strong relationship with FDA/EMEA would be preferable since the data isn’t clean. Added to that the high possibility of needing an additional trial and you want someone who can do that well and do so quickly.
3. InterMune has the option to convert the Roche profit share into a straight royalty. I could foresee them either deciding to do so now as the trial costs will be getting more significant with Phase 2 starting and likely multiple exploratory combo trials. Another option less likely but not out of the realm of possibility is bring in a third party to take on InterMune’s development cost in exchange for some of the profits down-the-road.
4. Dan Welch used the plural in potential partnerships for molecules. The Roche deal was described as similar for follow-on molecules. My understanding is if Roche declines on a molecule InterMune is free to develop it. We know of at least one molecule with a better profile (preclinically anyway) then 191. I don’t think Roche really wants a competing molecule from this partnership. I could see a deal (perhaps in conjunction with InterMune converting to a royalty) where Roche gains all rights to all HCV Protease molecules in exchange for a good chunck of cash and/or improved economics/cost reductions.
5. I don’t think InterMune is in position to partner the Helicase program at this stage so I think it is one thing they will keep going on their own.
IMO I think the drug provides some clinical benefit in some IPF patients, a small but meaningful percentage. I happen to be of the belief that the drug (at some doses) can help a wide range of patients from earlier disease state to even much more advanced then studied in this trial. The big problem is I don’t think anyone can predict who will benefit from the drug at the current time.
Primary End Point (change in percent predicted Forced Vital Capacity):
Capacity 1 Missed p=0.501. Capacity 2 Hit p=001
. Of note at weeks 12, 24, 36 and 48 both Capacity 1 and 2 had significant P values in FVC (except week 12 Capacity two which was .061)
. Repeat measures analysis over full trial length (retrospective) Capacity 1 p= 0.004, 2 p= 0.001 pulled p < 0.001
Secondary End Points
. Company plans to pursue EMEA and FDA filings at this time. When asked if will have dialog with FDA about whether another trial would be needed indicated at this time plan is to proceed with current package.
. General safe and well tolerated AE’s consistent with previous Pirfenidone trials (Rash, Stomach upset)
. No reason given for difference in trial out comes. Mentioned drug discontinuation unlikely to factor and demographics similar in two trials. Some possible areas for further exploration include different sites used for the two trials, placebo group behaving different than historical in Capacity 1 in the later weeks of the trial, and a sicker patient population group in Capacity 1 (specifically Oxygen use 2x that of Capacity 2 and baseline 6MWT about 10% lower than Capacity 2)
Some Speculations (Opinion only do not take as anything else)
1. My guess is it is likely to pass an FDA panel review but I am uncertain on FDA review. I think we get one or the other (FDA or EMEA) to approve with current data perhaps with Phase 4 or label restriction. I don’t know how easy a sale it would be I would push for a Phase 4 Pirfenidone+NAC perhaps 4 arm trial (assuming there is enough preclinical work/safety info to rationalize it). I am not sure if the two are antagonistic, synergistic (or something in between) but I imagine if approved patients would likely be on the combo so it is in the interest of everyone to know. If past trials are an indication and they are somewhat complimentary perhaps it gives a more meaningful result.
2. I put likelyhood of InterMune partnering Pirfenidone in some form as quite high. Keeping in mind that they have several analogs in various stages of preclinical development. I see a package partnership (if not the whole company). I would think a bigger Pharma or company with a strong relationship with FDA/EMEA would be preferable since the data isn’t clean. Added to that the high possibility of needing an additional trial and you want someone who can do that well and do so quickly.
3. InterMune has the option to convert the Roche profit share into a straight royalty. I could foresee them either deciding to do so now as the trial costs will be getting more significant with Phase 2 starting and likely multiple exploratory combo trials. Another option less likely but not out of the realm of possibility is bring in a third party to take on InterMune’s development cost in exchange for some of the profits down-the-road.
4. Dan Welch used the plural in potential partnerships for molecules. The Roche deal was described as similar for follow-on molecules. My understanding is if Roche declines on a molecule InterMune is free to develop it. We know of at least one molecule with a better profile (preclinically anyway) then 191. I don’t think Roche really wants a competing molecule from this partnership. I could see a deal (perhaps in conjunction with InterMune converting to a royalty) where Roche gains all rights to all HCV Protease molecules in exchange for a good chunck of cash and/or improved economics/cost reductions.
5. I don’t think InterMune is in position to partner the Helicase program at this stage so I think it is one thing they will keep going on their own.
IMO I think the drug provides some clinical benefit in some IPF patients, a small but meaningful percentage. I happen to be of the belief that the drug (at some doses) can help a wide range of patients from earlier disease state to even much more advanced then studied in this trial. The big problem is I don’t think anyone can predict who will benefit from the drug at the current time.
Join the InvestorsHub Community
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.






