Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
I think we'll hear this week from the FDA.
Not only that, but maybe even more importantly (short term) the Agenda Trial results...
...those who sell off if a denial of Genasense for the treatment CLL is announced will give up some really cheap shares for those of us who know that the Agenda Trial results are just right around the corner. Thats where this company will make its explosion onto the scene.
Genasense in the treatment of melanoma. That's the ticket.
Nice first post...
...the majority of the people here have done extensive DD on this company. Everyone realizes, fully, the financial situation with Genta.
In the end, it's all about the drugs.
My money, personally, is on the Agenda Trial and a partnership.
Those waiting for news:
Please read this article.
http://www.bioportfolio.com/news/btech_040802_2.htm
Genta and ImClone: The Tortoise and the Hare
In the wake of ImClone's (NASDAQ: IMCL) troubled Erbitux FDA application, biotech companies are sure to be more careful about hyping their developmental drugs. ImClone did a poor job of managing the expectations of its investors. ImClone took the fastest and most risky path towards FDA approval, attempting to have Erbitux approved based on phase II data.
Like ImClone, much of Genta's (NASDAQ: GNTA) future is resting on whether its lead drug, Genasense, is approved by the FDA in the next two years. But Genta has taken the opposite approach to seeking regulatory approval. Like Erbitux, Genasense is an innovative therapeutic that has the potential to treat a broad variety of cancers. Both drugs work extremely well in combination with existing forms of chemotherapy, and both drugs have the potential to be blockbusters. Genasense is an antisense therapeutic that blocks production of the Bcl-2 protein, a protein that protects cells from apoptosis (programmed cell death). Many tumor cells make excess Bcl-2 in order to avoid apoptosis.
In contrast to zero phase III trials used by ImClone prior to regulatory submission, Genta is currently conducting three phase III trials for malignant melanoma, multiple myeloma, and chronic lymphocytic leukemia (CLL). Genta has taken an extremely conservative approach to informing investors about Genasense trial progress. In contrast to ImClone's propensity to highlight even the smallest bit of positive trial data, Genta has kept quiet, asking investors to wait for the complete phase III data.
Genta's strategy is not without negative consequences. The lack of significant news about the progress of Genasense trials has invited some degree of speculation, often negative. This week, Genta's CEO Ray Warrell declined to explain why the company had enrolled more patients in the CLL phase III trial. Analysts feared that extra patients could mean that the trial wasn't going well, and this fear led to a 16.5% drop in Genta's stock price last Thursday. The reaction prompted Warrell to hold a conference call Friday morning. In the call, Warrell explained that the decision to add more patients had nothing to do with clinical results, and had in fact been made last summer before any clinical results were even known. Beyond this, Warrell stuck to the company's plan, giving no more indication, positive or negative, about the progress of the phase III trials.
Expect to see companies following the tortoise rather than the hare approach to developing drugs, at least in the near future. If Genasense is approved in 2003 or 2004, Genta's approach will be held up as an example of successful long-term strategy. Such conservatism will not always be
appropriate, but there is plenty of room in between the ImClone and Genta approaches for companies to seek speedy regulatory approval without taking undue risks.
Go listen to the conference call.
*huge eye roll*
If anyone thinks this drug has been approved they are morons. Period. You'd be seeing all kinds of price movement on this stock and your GNTA PR section of your trading desk would be loaded with news.
This is a fabricated image and was done as a joke. It's fake.
The message board is a cesspool.
Don't post moronic things...
...to avoid being called a moron.
I understand...
...I wasn't speaking to you specifically, I just happened to hit reply after reading your post.
Good lord people...
...use your heads for something other than a hatrack. When this news breaks, there will be a significant movement in this stock (positively or negatively) probably before anyone on here knows what's happening...shortly thereafter (like a few minutes) followed by a PR from the company.
That image is a fake and it was a joke, don't be morons.
Obama's Budget & Healthcare Reform
...includes 6 Billion Dollars for Cancer Research.
Absolutely...
...the Agenda Trial is where my money resides and I don't expect to hear anything on that for another 6 months.
I do expect to hear something on partnerships sooner than that.
Same thing happened with Genasense NDA!
I'm not sure...
...but there is some theory out there, and I'm totally not sure if it's been proven or not, that A short seller has to borrow stock in order to sell it short. If no
stock is available to be borrowed, he can't go short. Short sellers
drive the price down, so some of the posters here who are long on GNTA
want to make it harder for people to go short. They think they can do
that by setting sell orders at 1.50. They think setting such an order
will tie their stock up so their brokers can't loan them out to short
sellers.
I'll honestly tell you that I have no clue if this is true or not, but more of a case study to have some fun and see what happens.
Everyone set a sell limit order...
...of all of your shares at 2 dollars.
People are getting ready...the FDA announcement is drawing closer.
I gave him a link.
vawedo...
...there was an appeal of that report. It appears the EMEA asked for an additional clinical trial, which Genta is giving them with the Agenda Trial.
http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/07-19-2007/0004629263&EDATE
Vawedo...
...I can't respond to you on pm because I don't have an account here. What you linked is an old lawsuit, which has been settled. You may remember from the conference call this being discussed. It's a closed issue.
Research the Agenda Trial.
I think this is the appeal you're looking for.
http://www.medicalnewstoday.com/articles/133015.php
The PR is dated December 15th, 2008. I don't think anyone is exactly clear on when the appeal was officially submitted. Genta was to meet, formally, with FDA leadership to understand the issues associated with the NDA (reason(s) for non-approval) and address those issues prior to submitting the appeal.
I think the appeal was probably submited late January, early February.
stocktrad, you need to do some research before you post.
Genasense.com is just a domain name that was purchased. There was never anything on it to begin with.
This is the second time I've had to point something that is very elementary due diligence on Genta.
Go back 600 posts on this board, make some popcorn, and start reading.
Well it's right there on their website, I'm not sure what was so elusive about it.
What the hell are you talking about? It's right here on the damn site as the most recent press release. Open your eyes.
http://www.genta.com/investor%20relations/pressreleases2.php?v=4&option=com_content&task=view&id=60&Itemid=&CID=423&SHID=&COID=
Genta management will host a conference call and live audio webcast to discuss financial results and corporate activities on February 13, 2009 at 4:30 pm ET. Participants can access the live call by dialing (877) 634-8606 (U.S. and Canada) or (973) 200-3973 (International). The access code for the live call is Genta Incorporated. The call will also be webcast live at http://www.genta.com/investorrelation/events.html. For investors unable to participate in the live call, a replay will be available approximately two hours after the completion of the call, and will be archived for 30 days. Access numbers for this replay are: (800) 642-1687 (U.S. and Canada) and (706) 645-9291 (International); conference ID number is 84733610.
What webcast?
Incorrect.
The CEO of Genta said that a successful Agenda Clinical Trial (which is being conducted to confirm a prior phase III study's positive results for the FDA and EMEA) was "highly likely" based upon "overwhelming" data.
Further, prior to the conference call taking place, it was communicated that an archived file of the conference call would only be available on the site for a certain number of days.
OF COURSE!
The economic stimulus measure signed by Obama on February 17 included a huge, two-year infusion of about $10 billion for the National Institutes of Health, which funds research, of which about $1.26 billion is expected to go for cancer research.
"What will be more important is a sustained growth in the national investment in cancer research because we can only do so much with a short-term bolus of money," Dr. Richard Schilsky of the University of Chicago, president of the American Society of Clinical Oncology, said in a telephone interview.
"If the money goes away, then a lot of projects will have been started but not many will have been completed," he added.
CONNECT THE DOTS TO GENTA AND THE NCI:
Genasense® (oblimersen sodium) Injection has been in clinical trials since 1995 in the U.S., Europe and Australia, with efficacy and safety data from Phase 1, Phase 2 and Phase 3 clinical trials. More than 2,200 patients have entered randomized trials (one-half of whom were controls) and approximately 500 patients have been treated in non-randomized Phase 2 trials sponsored by Genta, through a long-standing Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI), or the European Organization for the Research and Treatment of Cancer (EORTC).
Don't think for a second that Genta won't be around long enough to complete the AGENDA Clinical Trial and see it through the NDA process. Genta and the NCI have had an agreement on Genasense since 1995. They're not going to turn their backs on this relationship when the Agenda trial is right at the finish line.
The economic stimulus measure signed by Obama on February 17 included a huge, two-year infusion of about $10 billion for the National Institutes of Health, which funds research, of which about $1.26 billion is expected to go for cancer research.
"What will be more important is a sustained growth in the national investment in cancer research because we can only do so much with a short-term bolus of money," Dr. Richard Schilsky of the University of Chicago, president of the American Society of Clinical Oncology, said in a telephone interview.
"If the money goes away, then a lot of projects will have been started but not many will have been completed," he added.
CONNECT THE DOTS TO GENTA AND THE NCI:
Genasense® (oblimersen sodium) Injection has been in clinical trials since 1995 in the U.S., Europe and Australia, with efficacy and safety data from Phase 1, Phase 2 and Phase 3 clinical trials. More than 2,200 patients have entered randomized trials (one-half of whom were controls) and approximately 500 patients have been treated in non-randomized Phase 2 trials sponsored by Genta, through a long-standing Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI), or the European Organization for the Research and Treatment of Cancer (EORTC).
Don't think for a second that Genta won't be around long enough to complete the AGENDA Clinical Trial and see it through the NDA process. Genta and the NCI have had an agreement on Genasense since 1995. They're not going to turn their backs on this relationship when the Agenda trial is right at the finish line.
We need to start doing some research on Genta's relationship with the US NCI. It looks like their about to get a boat load of money for cancer research.
http://www.boston.com/news/nation/washington/articles/2009/02/25/obama_cancer_cure_vow_requires_more_funds_experts/?rss_id=Boston.com+--+Latest+news
I can find something that mentions a research and development agreement with the NCI in 2005 for Genasense...I feel like Genta is about to get a financial shot in the arm from the National Cancer Institute.
http://www.drugs.com/nda/genasense_051229.html
Genasense inhibits production of Bcl-2, a protein made by cancer cells that is thought to block chemotherapy-induced apoptosis (programmed cell death). By reducing the amount of Bcl-2 in cancer cells, Genasense may enhance the effectiveness of current anticancer treatment. Genta is pursuing a broad clinical development program with Genasense to evaluate its potential to treat various forms of hematologic cancers and solid tumors. In addition, Genta has established a Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI).
Genasense® (oblimersen sodium) Injection has been in clinical trials since 1995 in the U.S., Europe and Australia, with efficacy and safety data from Phase 1, Phase 2 and Phase 3 clinical trials. More than 2,200 patients have entered randomized trials (one-half of whom were controls) and approximately 500 patients have been treated in non-randomized Phase 2 trials sponsored by Genta, through a long-standing Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI), or the European Organization for the Research and Treatment of Cancer (EORTC).
Clinical Trials Tab:
http://www.genta.com/clinical_development/genasense.php?v=6&PHPSESSID=82ae55518847e22a3896f10a2a83cb25
Buy pressure heating up
Weekends knock 2 days off FDA approval time...
...I don't think people want to be left out of something comes out over the weekend.
Every day, the decision looms closer.
Gonna run guys...that shake was weak!
GNTA will show them how it's done
Looking good here folks...
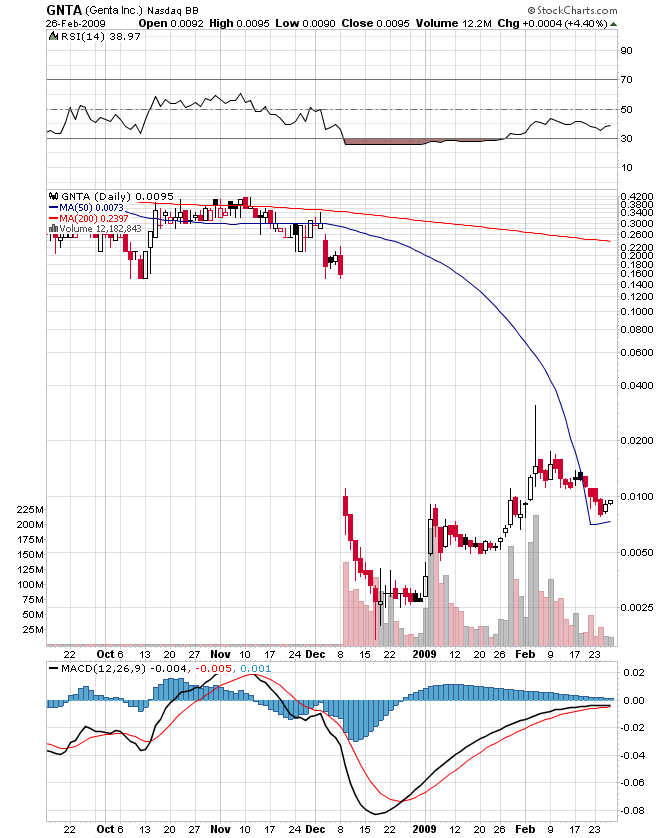
The same thing can be said for many companies...even major big board stocks...look at BAC.
Just a little over a year ago, if you invested 10,000 dollars at 50 dollars per share, you'd have a lot of money in there. If you looked at it now...it wouldn't be too much.
AWESOME NEWS! Great DD again, nutriman!
For those who don't know, the M.D. Anderson Cancer Center is conducting Genta's Agenda Phase III clinical trial.
Do I hear a penny today?
It's obvious that the momentum is flipping from sell to buy. We could see it today...and if we do....hoooooold on.
Mix in the appealed NDA and Tesetaxel...
...with the Agenda Trial and you have a concoction for an explosive security.
Here it is if someone could sticky it.
It has all the information you need to know on why there is so much excitement and interest in Genta (GNTA).
http://investorshub.advfn.com/boards/read_msg.aspx?message_id=35872172
