Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
$BSEM –Hope it hits $15 soon after big ER beat
$BSEM on watchlist after massive earnings beat- improved to 95%, while 1Q revenue was 71 times higher than the year-old period, blowing past our estimates and posting its first profit. https://s27.q4cdn.com/906368049/files/News/2024/Zacks_SCR_Research_05152024_BSEM_Sorensen.pdf
$BSEM compete with $VCEL $MRNA $VRTX $BGNE $VCEL $VRTX $SGMO $BCLI $GMDA
Update: VCEL closed at $49.20 per share hitting a high of $49.91/share. Currently our 12k shares of VCEL are fighting it out with 755 shares of NVDA for highest market value in a TDA taxable account. Both are LTCG with cost basis already removed.
They lowered guidance in the conference call.
Due to the conversion rate for MACI
Dead money for the remainder of 2022
https://seekingalpha.com/article/4555456-vericel-corporation-vcel-q3-2022-earnings-call-transcript
Fezzywig2008 Lost it all in this piece
Vericel stock price target cut to $36 from $44 at Truist
VCEL is trading down because they didn't raise guidance even though they beat on revenue.
The Q2 guidance was lower than what analyst had. (my opinion, based on a question from an analyst question. I didn't calculate it)
Vericel GAAP EPS of -$0.15 misses by $0.03, revenue of $36.07M beats by $1.76M
Wed, May 4, 2022, 7:55 AM
First Quarter Total Net Revenue of $36.1 Million
Conference Call Today at 8:30am Eastern Time
CAMBRIDGE, Mass., May 04, 2022 (GLOBE NEWSWIRE) -- Vericel Corporation (NASDAQ:VCEL), a leader in advanced therapies for the sports medicine and severe burn care markets, today reported financial results and business highlights for the first quarter ended March 31, 2022.
First Quarter 2022 Financial Highlights
Total net revenue of $36.1 million, compared to $34.6 million in the first quarter of 2021
MACI® net revenue of $26.0 million, Epicel® net revenue of $9.9 million, and NexoBrid® revenue of $0.2 million related to the U.S. Biomedical Advanced Research and Development Authority (“BARDA”) procurement for emergency response preparedness
Gross margin of 65%, compared to 66% in the first quarter of 2021
Net loss of $7.1 million, or $0.15 per share, compared to $3.3 million, or $0.07 per share, in the first quarter of 2021
Non-GAAP adjusted EBITDA of $3.2 million, compared to $4.6 million in the first quarter of 2021
Operating cash flow of $3.5 million
As of March 31, 2022, the Company had approximately $130 million in cash, restricted cash and investments, and no debt
Business Highlights and Updates
Double-digit growth in surgeons taking MACI biopsies compared to the first quarter of 2021, with the second highest monthly biopsies in March 2022 since the launch of MACI
Growth of more than 20% in burn centers treating patients and taking Epicel biopsies compared to the first quarter of 2021, with a record monthly high in Epicel biopsies in March 2022
Remain on track for a planned mid-year 2022 resubmission of the NexoBrid Biologics License Application to the FDA, and
Expanded the Company’s commercial leadership team with the appointment of Mike Gilligan as Vice President, MACI National Sales
“The Company executed well in the first quarter and we remain on track to deliver another year of significant revenue growth, margin expansion, and operating cash flow driven by continued strong results for both MACI and Epicel,” said Nick Colangelo, President and CEO of Vericel. “We also continue to advance important regulatory and clinical programs across both our sports medicine and burn care franchises as we remain on track for a mid-year resubmission of the NexoBrid BLA and for planned discussions with the FDA to review both the MACI arthroscopic and ankle development programs, initiatives that we believe will support continued strong growth in the years ahead.”
2022 Financial Guidance
The Company reaffirmed financial guidance for full-year 2022
Total net revenue for 2022 expected to be in the range of $178 to $189 million
MACI revenue expected to be in the range of $132 to $141 million
Epicel revenue expected to be in the range of $45.5 to $47.5 million
Gross margin expected to be approximately 70%
Adjusted EBITDA margin expected to be approximately 21%
First Quarter 2022 Results
Total net revenue for the quarter ended March 31, 2022 increased 4% to $36.1 million, compared to $34.6 million in the first quarter of 2021. Total net product revenue for the quarter increased 7% and included $26.0 million of MACI (autologous cultured chondrocytes on porcine collagen membrane) net revenue and $9.9 million of Epicel (cultured epidermal autografts) net revenue, compared to $23.8 million of MACI net revenue and $9.8 million of Epicel net revenue, respectively, in the first quarter of 2021. Total net revenue for the quarter also included $0.2 million of revenue related to the procurement of NexoBrid (concentrate of proteolytic enzymes enriched in bromelain) by BARDA for emergency response preparedness, compared to $0.9 million in the first quarter of 2021.
Gross profit for the quarter ended March 31, 2022 was $23.5 million, or 65% of net revenue, compared to $23.0 million, or 66% of net revenue, for the first quarter of 2021.
Total operating expenses for the quarter ended March 31, 2022 were $30.7 million, compared to $26.3 million for the same period in 2021. The increase in operating expenses was primarily due to higher stock-based compensation expense.
Net loss for the quarter ended March 31, 2022 was $7.1 million, or $0.15 per share, compared to a net loss of $3.3 million, or $0.07 per share, for the first quarter of 2021.
Non-GAAP adjusted EBITDA for the quarter ended March 31, 2022 was $3.2 million, or 9% of net revenue, compared to $4.6 million, or 13% of net revenue, for the first quarter of 2021. A table reconciling non-GAAP measures is included in this press release for reference.
As of March 31, 2022, the Company had approximately $130 million in cash, restricted cash and investments, and no debt.
We still have 15k shares of VCEL with cost basis of $3.32. We surely are not down 96.2% from all time high; rather we are up 1066.85% at close of $38.75.
Assuming VCEL gets the NexoBrid approval this year, I can see your 1066.85% becoming 5000% by end 2023. Not kidding here, all VCEL products are basically without competition and without generic pathways.
What do you see re direction of market the rest of year, AMRN in particular?
I only have a small position in AMRN........ its prospects seem murky to me, they don't seem to have stemmed generic erosion in the USA, and its always the USA that gets reflected in price.... of course PFE signing on for Canadian sales suggests interest. As for the market, who knows, but one would think recovery to some norm is inevitable. The most clear winners like VCEL should lead.
"...surely an error..."!!
We still have 15k shares of VCEL with cost basis of $3.32. We surely are not down 96.2% from all time high; rather we are up 1066.85% at close of $38.75.
JNJ and CDMO continue to do well.
What do you see re direction of market the rest of year, AMRN in particular?
—Down 96.2% from its all-time closing high of $1410.00 on Sept. 24, 1997
Surely an error in that it fails to calculate for one or two reverse splits by ASTM which was the former ticker until VCEL basically purchased it as a shell.
I had the wrong title of article from Dow Jones. The correct title is Data Talk, 3/18/21 at 12:05 pm:
“Vericel Up Over 14%, on Track for Highest Close Since November 2011....
—Up 73.58% year to date( that was yesterday)
—Down 96.2% from its all-time closing high of $1410.00 on Sept. 24, 1997.
—Up 557.67% from 52 weeks ago(3/19/20 closed at $8.15....(more)”
Dow Jones Fact Set: VCEL advance predicated on addition to Small Cap index --- whose index, I have no current idea. 3/18/2021 is date of article.
interesting set of stats....all time high for Vericel said to be in excess of $1000/share many years ago.
Hey there north, long time.........
Now trading around $57 .......... I went to Twitter wondering if someone might know of an explanation - and I found 3 different ones, which you may find amusing:
"StockTwits Trending Alert: Trading recent interest in AASTROM BIOSCIENCES INC. $VCEL"
StockTwits Trending Alert: Trading recent interest in AASTROM BIOSCIENCES INC. COMM $VCEL https://t.co/3y1SdMW2hA
— Quantcha Trade Ideas (@QuantchaIdeas) March 19, 2021
High share price increase pre-market today. Goes with the necessary buying. Note also the new SEC rule effective today.
Vericel Set to Join S&P SmallCap 600 Replacing QEP Resources
Hey Doc Vcel is a great company and so is Celz...future partners maybe? (Patel, Henry)
https://pubmed.ncbi.nlm.nih.gov/27059887/
Fizzywig use to honk on my Bobo!
Hopefully we have multiple bidders. I have no idea what kind of offer(s) May be on the table. I am seeing prices from some messages on stocktwits from 63 to over $100 per share and then combinations with stock.
Anyone have thoughts on today’s news? Buyout at a much higher price, perhaps?
COO sells 30,000
Chief Medical Officer - sells more than that
VCEL Vericel Stephens & Co. Initiates Coverage On Overweight 31.00
Ed Brown is now the largest stockholder. 3,910,122
DEERFIELD cut their position in half
Vericel Reports Record Third Quarter Revenues and Net Income
8:02 am ET November 5, 2020 (Globe Newswire) Print
Total Net Revenues of $32.3 Million and Net Income of $3.6 Million
Conference Call Today at 8:30am Eastern Time
Vericel Corporation (NASDAQ:VCEL), a leader in advanced therapies for the sports medicine and severe burn care markets, today reported financial results and business highlights for the third quarter ended September 30, 2020.
Third Quarter 2020 Financial Highlights
-- Total net revenues of $32.3 million, compared to $30.5 million in the third quarter of 2019;
-- MACI net revenue of $24.4 million, Epicel net revenue of $6.7 million and NexoBrid revenue of $1.2 million related to the U.S. Biomedical Advanced Research and Development Authority (BARDA) procurement for national response preparedness;
-- Gross margin of 70%, compared to gross margin of 69% in the third quarter of 2019;
-- Net income of $3.6 million, or $0.08 per share, compared to $3.5 million, or $0.07 per share, in the third quarter of 2019;
-- Non-GAAP adjusted EBITDA of $6.7 million, compared to $6.8 million in the third quarter of 2019;
-- Operating cash flow of $4.6 million; and
-- Cash and investments of $85.5 million as of September 30, 2020, compared to $79.0 million as of December 31, 2019, and no debt.
Business Highlights and Updates
-- Reported record third quarter MACI revenue and total revenues, and the second highest quarterly Epicel revenue in history;
-- Achieved double-digit growth in MACI revenue, implants and biopsies in the third quarter, including a record monthly high for biopsies in September;
-- Announced the first delivery of NexoBrid to BARDA for emergency response preparedness; and
-- Announced that the FDA accepted for review the Biologics License Application for NexoBrid for the treatment of severe thermal burns, with a PDUFA goal date of June 29, 2021.
"Our company executed exceedingly well during the third quarter as we generated stronger than expected financial results, drove strong commercial performance for MACI and Epicel, and achieved important milestones towards our goal of obtaining marketing approval of NexoBrid in the United States," said Nick Colangelo, President and CEO of Vericel. "Our third quarter results demonstrated the strength of our business across several measures and, while uncertainties related to COVID-19 remain, we are highly confident in the underlying fundamentals of our business and we remain on track to deliver strong revenue and profit growth in the years ahead."
Third Quarter 2020 Results
Total net revenues for the quarter ended September 30, 2020 increased 6% to $32.3 million, compared to $30.5 million in the third quarter of 2019. Total net product revenues for the quarter included $24.4 million of MACI (autologous cultured chondrocytes on porcine collagen membrane) net revenue and $6.7 million of Epicel (cultured epidermal autografts) net revenue compared to $20.6 million of MACI net revenue and $9.9 million of Epicel net revenue, respectively, in the third quarter of 2019, and $1.2 million of revenue related to the procurement of NexoBrid (concentrate of proteolytic enzymes enriched in bromelain) by BARDA for emergency response preparedness.
Gross profit for the quarter ended September 30, 2020 was $22.5 million, or 70% of net revenues, compared to $21.2 million, or 69% of net revenues, for the third quarter of 2019.
Total operating expenses for the quarter ended September 30, 2020 were $19.0 million, compared to $18.1 million for the same period in 2019. The increase was primarily driven by incremental employee expenses related to the MACI sales force expansion.
Vericel's net income for the quarter ended September 30, 2020 was $3.6 million, or $0.08 per share, compared to $3.5 million, or $0.07 per share, for the third quarter of 2019.
Non-GAAP adjusted EBITDA was $6.7 million for the quarter ended September 30, 2020, compared to $6.8 million in the third quarter of 2019. A table reconciling non-GAAP measures is included in this press release for reference.
As of September 30, 2020, the company had $85.5 million in cash and investments, compared to $79.0 million as of December 31, 2019, and no debt.
Conference Call Information
Today's conference call will be available live at 8:30am Eastern Time and can be accessed through the Investor Relations section of the Vericel website at http://investors.vcel.com/events-presentations. A slide presentation with highlights from today's conference call will be available on the webcast and in the Investor Relations section of the Vericel website. Please access the site at least 15 minutes prior to the scheduled start time in order to download the required audio software, if necessary. To participate in the live call by telephone, please call (877) 312-5881 and reference Vericel Corporation's third-quarter 2020 investor conference call. If calling from outside the U.S., please use the international phone number (253) 237-1173.
If you are unable to participate in the live call, the webcast will be available at http://investors.vcel.com/events-presentations until November 5, 2021. A replay of the call will also be available until 11:00am (EDT) on November 12, 2020 by calling (855) 859-2056, or from outside the U.S. by calling (404) 537-3406. The conference ID is 5426489.
Vericel Announces Preliminary Third Quarter 2020 Total Net Revenues of $32 Million
October 14, 2020 at 8:00 AM EDT
PDF Version
Double-Digit Revenue, Implant and Biopsy Growth for MACI in the Quarter and Record Monthly Biopsies in September
Approximately $4.6 Million of Operating Cash Flow for the Quarter
CAMBRIDGE, Mass., Oct. 14, 2020 (GLOBE NEWSWIRE) -- Vericel Corporation (NASDAQ:VCEL), a leader in advanced therapies for the sports medicine and severe burn care markets, today announced preliminary financial results for the quarter ended September 30, 2020.
Preliminary total net revenues for the third quarter are expected to be approximately $32 million, including approximately $24.2 million of MACI® (autologous cultured chondrocytes on porcine collagen membrane) net revenue, approximately $6.7 million of Epicel® (cultured epidermal autografts) net revenue, and approximately $1.2 million of revenue related to the procurement of NexoBrid® (concentrate of proteolytic enzymes enriched in bromelain) by the U.S. Biomedical Advanced Research and Development Authority (BARDA) for emergency response preparedness.
The company generated approximately $4.6 million of operating cash flow in the third quarter. As of September 30, 2020, the company had approximately $85.5 million in cash and investments and no debt, compared to $79.0 million as of December 31, 2019.
“We are very pleased with our third quarter results as we generated consistent double-digit growth in revenue, implants and biopsies for MACI and achieved a record monthly high for biopsies in September,” said Nick Colangelo, President and CEO of Vericel. “Moreover, the robustness of our business model was demonstrated as we generated positive operating cash flow for the quarter. While considerable uncertainties related to COVID-19 remain, given the strength of our patient pipeline, we expect to maintain strong MACI growth in the fourth quarter. We look forward to providing further updates to investors during our upcoming virtual Analyst and Investor Day webcast and our third quarter earnings call.”
As previously announced, the company will host a virtual Analyst and Investor Day on October 16, 2020, at 9:00am Eastern Time. The company also will host a webcast and conference call to discuss its third quarter 2020 financial results and business highlights on November 5, 2020, at 8:30am Eastern Time. Webcast information can be found on the events and presentation section of the Investor Relations website at https://investors.vcel.com/events-presentations.
About Vericel Corporation
Vericel is a leader in advanced therapies for the sports medicine and severe burn care markets. The company markets two cell therapy products in the United States. MACI (autologous cultured chondrocytes on porcine collagen membrane) is an autologous cellularized scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. Epicel (cultured epidermal autografts) is a permanent skin replacement for the treatment of patients with deep dermal or full-thickness burns greater than or equal to 30% of total body surface area. The company also holds an exclusive license for North American commercial rights to NexoBrid, a registration-stage biological orphan product for debridement of severe thermal burns. For more information, please visit the company’s website at www.vcel.com.
Epicel® and MACI® are registered trademarks of Vericel Corporation. NexoBrid® is a registered trademark of MediWound Ltd. and is used under license to Vericel Corporation. © 2020 Vericel Corporation. All rights reserved.
Preliminary and Unaudited Nature of Reported Results
Our revenue expectations for the third quarter, as well as our estimates concerning operating cash flow and cash and investments are preliminary, unaudited and are subject to adjustment in the course of our ongoing internal control and review procedures.
Delcath Systems appoints Gerard Michel as CEO, John Purpura as COO 16:12 DCTH, VCEL Delcath Systems (DCTH) announced that the board appointed Gerard Michel as CEO, effective October 1. Michel will also serve as a member of the Delcath Systems board. In his most recent role, Michel was the CFO and VP of corporate development at Vericel (VCEL). Michel was a member of the executive team that restructured Vericel. In addition to Michel's appointment as CEO, John Purpura, was appointed as COO. Purpura's experience in areas of regulatory affairs, manufacturing and distribution have been a critical component of preparing Delcath for its planned new drug application, or NDA, resubmission to the FDA in mid-2021.
Read more at:
https://thefly.com/n.php?id=3169037
Don´t trust the Trust.
Trust Co > Sun Trust > Truist
Does that mean they can no longer be Trusted ?
lol
Realfast thanks. Everything should be ready to commence sales by then. It is an unsufferable experience to wait for so long on a product that has been on sale in Europe for ages which efficacy has been proven.
June 29th is when the FDA will reply to the request for approval.
If they say yes, then VCEL can sell NexoBrid in the USA.
Sales would be in Q3 of 2021
it sucks!
Realfast does it mean that from 29th June Nexobrid may be marketed? or when is it projected to be launched? Thanks
Vericel Corporation (NASDAQ: VCEL) today announced that the U.S. Food and Drug Administration (FDA) has accepted for filing the recently submitted Biologics License Application (BLA) for NexoBrid® (concentrate of proteolytic enzymes enriched in bromelain) for eschar removal (debridement) in adults with deep partial-thickness and/or full-thickness thermal burns. The FDA assigned a Prescription Drug User Fee Act (PDUFA) target date of June 29, 2021. In addition, the FDA communicated that it is not currently planning to hold an advisory committee meeting to discuss the application.
“The FDA’s acceptance of the NexoBrid BLA for review represents another important milestone toward our goal of providing a new standard of care for eschar removal in patients with severe burns,” said Nick Colangelo, President and CEO of Vericel. “We look forward, together with MediWound, to working with the FDA during the BLA review process as we seek marketing approval for NexoBrid in the United States.”
Sharon Malka, CEO of MediWound added, “The acceptance of the NexoBrid BLA is a major milestone for MediWound and it is gratifying to know NexoBrid is one step closer to being available to help burn victims in the United States. We thank all of the investigators, their teams, our employees and all our partners, especially BARDA and Vericel, for their commitment to the program.”
Vericel will host a virtual Analyst and Investor Day on Friday, October 16, 2020, from 9:00 a.m. - 11:00 a.m. EST which will focus on NexoBrid. In addition to a general corporate update, Vericel executives will facilitate discussions with burn surgeon thought leaders on current burn debridement practices and how NexoBrid, upon approval, could change the current treatment paradigm for debridement of severe thermal burns.
Thanks Realfast. Do you think Californian fires has anything to do with the spike? We may have NEXOBRID FDA news late this month.
currently in a breakout range
ma pull back after a good run
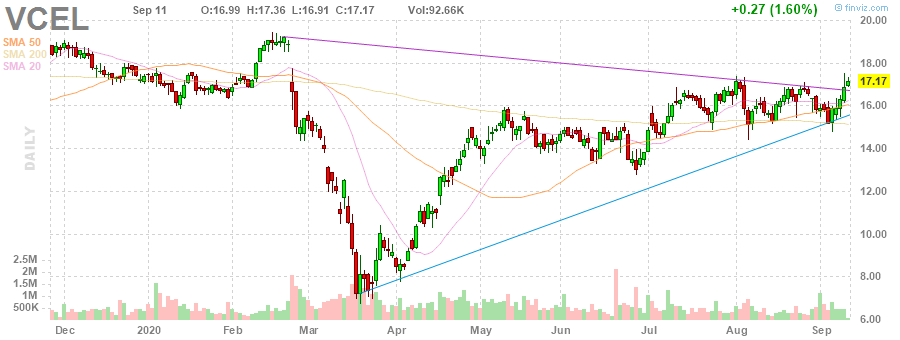
CONFIRMED breakout above 17.26, no resistance in area just above.
Type: True breakout from double resistance.
Target: 18.79
https://www.stockconsultant.com/consultnow/basicplus.cgi?symbol=VCEL
Hi Realfast. I find Vericel a very positve behaviour on its stock price moving up nicely like two years ago ascent. Do you think new funds and private investors are buying stock in the open market? Thank you.
On September 1, 2020, Gerard Michel notified Vericel Corporation (the “Company”) of his intention to resign as the Company’s Chief Financial Officer and Vice President of Business Development in order to join a public life sciences company as its Chief Executive Officer. The effective date of Mr. Michel’s resignation is September 30, 2020. The Company will immediately commence an external search for its next Chief Financial Officer.
On September 30, 2020, Mr. Michel will cease serving as the Company’s principal financial officer and principal accounting officer. On September 5, 2020, the Company’s current Corporate Controller, Sandra Pennell, age 41, was appointed to serve as the Company’s principal financial officer and principal accounting officer effective upon Mr. Michel’s resignation. Ms. Pennell has served in her current position at the Company since January 2015. Prior to joining the Company, Ms. Pennell served as Manager of External Reporting and Research at ITC Holding Corporation from September 2009 through December 2014, and as Director of Accounting at Fluid Routing Solutions, Inc. from January 2008 through September 2009. Ms. Pennell is a Certified Public Accountant and worked over 5 years in public accounting. Ms. Pennell has a Bachelor of Science degree from the University of Illinois at Urbana-Champaign and a Masters of Accounting from the Gies College of Business at the University of Illinois at Urbana-Champaign.
MediWound and Vericel Announce Acceptance of the First Delivery of NexoBrid to BARDA for Emergency Response Preparedness
YAVNE, Israel and CAMBRIDGE, Mass., Aug. 25, 2020 (GLOBE NEWSWIRE) -- MediWound Ltd. (NASDAQ: MDWD) and its U.S. commercial partner Vericel Corporation (NASDAQ: VCEL) today announced that the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services, has accepted the first shipment of NexoBrid® (concentrate of proteolytic enzymes enriched in Bromelain) as part of its mission to build national preparedness for public health emergencies. The initial BARDA procurement of NexoBrid is valued at $16.5 million, which includes additional quarterly deliveries planned through the end of 2021. In addition, BARDA holds an option to procure additional quantities of NexoBrid through funding of up to $50 million.
“This first shipment of NexoBrid is another major milestone in our longstanding partnership with BARDA, and we look forward to delivering the full procurement by the end of 2021,” said MediWound Chief Executive Officer Sharon Malka. “The acceptance of the first delivery by BARDA during the ongoing COVID-19 pandemic underscores the importance of NexoBrid to U.S. national preparedness for the potential emergency treatment of large numbers of patients with severe thermal burns injuries.”
“The procurement of NexoBrid for our nation’s response preparedness is one of many key NexoBrid activities occurring in 2020,” said Nick Colangelo, President and Chief Executive Officer of Vericel. “This shipment, combined with the recent BLA submission and growing number of burn centers enrolled in the NexoBrid expanded access treatment protocol, bring us a step closer to ensuring that healthcare providers have both the supply and training needed to enzymatically debride burn patients, whether the need is due to individual accidents or a burn mass casualty event.”
On June 30, 2020, a Biologics License Application (BLA) was submitted to the U.S. Food and Drug Administration (FDA) seeking the approval of NexoBrid for eschar removal (debridement) in adults with deep partial-thickness and/or full-thickness thermal burns. While the BLA is being reviewed by the FDA, burn centers across the U.S. are treating burn patients under the NexoBrid expanded access (NEXT) protocol.
The procurement is a key milestone of the Project BioShield (PBS) contract between MediWound and BARDA which was signed in September 2015 with procurement initiated in January 2020. Under the PBS contract, BARDA provides funds and support for the advancement of the development and manufacturing of NexoBrid, as well as the procurement of NexoBrid as a medical countermeasure for mass casualty emergencies involving thermal burns. In May 2019, Vericel entered into exclusive license and supply agreements with MediWound to commercialize NexoBrid in North America. As part of the license agreement, Vericel and MediWound will equally split gross profits generated by BARDA’s initial $16.5 million procurement.
In October 2019, MediWound initiated the NEXT protocol, which is supported and funded by BARDA and enables the continued clinical use of NexoBrid for U.S. patients during the preparation and review of the NexoBrid BLA. NEXT is an open-label, single-arm treatment protocol which allows for the treatment of up to 150 burn patients with deep partial- and full-thickness thermal burns up to 30 percent of total body surface area. NEXT has been designed to be consistent with current real-life burn treatment practices in the U.S. and up to 30 U.S. burn centers are anticipated to participate. MediWound received FDA concurrence that patients can be treated under the NEXT protocol in a burn mass casualty incident that is not a declared national emergency. Therefore, this provides a mechanism for U.S. burn centers to treat patients and gain valuable experience using Nexobrid prior to FDA approval as well as making the product readily available for response to mass burn emergencies.
BARDA submitted a Pre-Emergency Use Authorization (PEUA) to FDA for the intended use of NexoBrid under an Emergency Use Authorization (EUA) during a declared emergency involving burn injuries. The availability of medical countermeasures (MCMs), such as Nexobrid, which mitigate preparedness gaps (like debridement and excision steps of burn care) will add to the United States Government’s armamentarium for treatment of mass burn casualties. The EUA is a mechanism by which the FDA can allow an unapproved medical product that qualifies as a mass casualty medical countermeasure to be used in a public health emergency.
unfortunately there isn't any catalyst until November, after the Elections. The only single news is MDWD acceptance by the FDA, as soon as this month, with the PDUFA FDA decision date assigned.
The bad news is that VCEL has several up gaps
up Jul-01-2020 13.85 to 13.905
up Apr-17-2020 11.22 to 11.42
up Apr-06-2020 8.66 to 8.79
I did not get a chance to listen to call but evidently no one was impressed . To bad , I thought institutions would like this company .
Vericel Q2 EPS $(0.18) Beats $(0.21) Estimate, Sales $20.00M Beat $18.87M Estimate
Vericel Announces Preliminary Second Quarter 2020 Financial Results and Provides Business Updates
8:30 am ET July 9, 2020 (Globe Newswire) Print
Vericel Corporation (NASDAQ:VCEL), a leader in advanced therapies for the sports medicine and severe burn care markets, today announced preliminary financial results for the quarter ended June 30, 2020, and provided business updates.
Preliminary Second Quarter Financial Results
-- Preliminary total net product revenues for the second quarter are expected to be approximately $20 million, including approximately $15 million of MACI (autologous cultured chondrocytes on porcine collagen membrane) net revenue and approximately $5 million of Epicel (cultured epidermal autografts) net revenue;
-- Total net product revenues for the second quarter decreased approximately 23% compared to the second quarter of 2019, with MACI net revenue decreasing approximately 27% and Epicel net revenue decreasing approximately 8% compared to the second quarter of 2019;
-- Total net product revenues, which declined approximately 78% in April and 32% in May compared to the same periods in 2019, increased approximately 30% in June compared to June 2019;
-- Total net product revenues for the first half of 2020 decreased approximately 2% compared to the first half of 2019, with MACI net revenue decreasing approximately 5% and Epicel net revenue increasing approximately 7% compared to the first half of 2019; and
-- As of June 30, 2020, the company had approximately $81 million in cash and investments and no debt.
Second Quarter Business Updates
-- MACI implants, which declined approximately 84% in April and 37% in May compared to the same periods in 2019, increased approximately 21% in June compared June 2019;
-- MACI biopsies declined approximately 79% in April and 22% in May compared to the same periods in 2019, and increased approximately 23% in June compared June 2019;
-- Approximately 70% of scheduled MACI cases that were cancelled in the first half of 2020 due to the COVID-19 pandemic have been rescheduled, with over 50% of the cancelled cases completed by the end of the second quarter;
-- Epicel graft volume, which declined 70% in April, increased approximately 20% in the May through June period compared to the same period in 2019; and
-- Epicel biopsies increased by approximately 6% in the second quarter compared to the second quarter of 2019.
"We saw a very strong recovery for MACI during the second quarter as restrictions on elective surgeries were lifted across the country," said Nick Colangelo, President and CEO of Vericel. "While considerable uncertainties related to COVID-19 remain, absent a significant resurgence in restrictions related to COVID-19 we expect growth for MACI in the third quarter, albeit at a more moderate rate compared to pre-COVID-19 levels given the decline in MACI biopsies in April and May. We will continue to monitor the evolving landscape and we look forward to updating investors on our second quarter earnings call."
The company will host a webcast and conference call to discuss its second quarter 2020 financial results and business highlights on August 5, 2020 at 8:30am Eastern Time.
We should see a response back on acceptance within 2 months.
I don't want to speculate on impact and timing for final approval.
Everyone seems to be looking forward to 2021 with this covid taking away 2020.
How long does it take the FDA to approve the license and if approved will it get any kind of a decent bump at all
|
Followers
|
139
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
8025
|
|
Created
|
11/08/03
|
Type
|
Free
|
| Moderators | |||
Vericel Corporation. (VCEL)
http://epicel.com/healthcare-professionals/what-is-epicel/epicel-is-fda-approved/
https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/ucm543860.htm

Click on Yellow to REVIEW FDA WEBSITE APPROVING MACI
http://www.maci.com/
Interesting FDA Transcript discussions on MACI going forward for widened age bracket and other body joints.
CLICK ON THE ABOVE LINK

CLICK ON THE ABOVE PICTURE TO GO INTO VCEL'S CAREER'S SITE
IXMYELOCEL-T
Our preapproval stage portfolio includes ixmyelocel-T, a unique patient-specific multicellular therapy derived from an adult patient’s own bone marrow which utilizes our proprietary, highly automated and scalable manufacturing system. Our proprietary cell manufacturing process significantly expands the mesenchymal stromal cells (MSCs) and M2-like anti-inflammatory macrophages in the patient’s bone marrow mononuclear cells while retaining many of the hematopoietic cells. These cell types are known to regulate the immune response and play a key role in tissue repair and regeneration by resolving pathologic inflammation, promoting angiogenesis, and remodeling ischemic tissue. The novelty and advantage of using ixmyelocel-T is the expansion of a unique combination of cell populations, including MSCs and M2-like macrophages, which secrete a distinct combination of angiogenic and regenerative factors, and possess the ability to remain anti-inflammatory in the face of inflammatory challenge.


Vericel develops, manufactures, and markets autologous cell-based therapies for patients with serious diseases and conditions. The company markets two cell therapy products in the United States. Carticel® (autologous cultured chondrocytes) is an autologous chondrocyte implant intended to treat cartilage defects in the knee in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure. Epicel® (cultured epidermal autografts) is a permanent skin replacement for the treatment of patients with deep dermal or full thickness burns greater than or equal to 30% of total body surface area. Vericel is also developing 2 additional cell products. MACI is a third generation autologous chondrocyte implant intended to treat cartilage defects in the knee. Ixmyelocel-T is a multicellular therapy intended to treat advanced heart failure due to ischemic dilated cardiomyopathy (DCM).
Developing autologous (patient’s own) cell therapies—with integrity
Vericel uses rigorous scientific methods to develop novel therapies for the treatment of patients with autologous (patient’s own) cells. In addition, personal integrity, team work, collaboration, and innovative technology are the foundations of our work. We seek to practice transparency in our clinical trials and research, and in our relationships with each other, our patients, and the investors who support us.
Management:
http://vcel.com/about-vericel/#Management
Clinical Research:
http://vcel.com/research-and-development/#cardioMyopathy
Recent News:
http://investors.aastrom.com/releases.cfm
http://www.4-traders.com/VERICEL-CORP-18635051/
http://finance.yahoo.com/q/h?s=VCEL+Headlines
Events and Presentations:
http://investors.aastrom.com/events.cfm
Analyst Coverage:
http://investors.aastrom.com/analysts.cfm
http://www.streetinsider.com/Analyst+Comments/Vericel+(VCEL)+MACI+Approval+Puts+Profitability+Within+Reach+-+Needham+%26+Company/12343070.html
https://www.thecerbatgem.com/2016/12/19/needham-company-llc-analysts-give-vericel-corp-vcel-a-9-00-price-target.html
https://sportsperspectives.com/2016/12/22/piper-jaffray-cos-begins-coverage-on-vericel-corp-vcel.html
http://www.4-traders.com/VERICEL-CORP-18635051/consensus/
http://seekingalpha.com/symbol/VCEL
http://seekingalpha.com/article/1228921-is-takeda-interested-in-aastrom-bio
Filings:
http://www.sec.gov/cgi-bin/browse-edgar?company=&match=&CIK=0000887359&filenum=&State=&Country=&SIC=&owner=exclude&Find=Find+Companies&action=getcompany
Share Structure:
See Filings
http://data.cnbc.com/quotes/vcel
http://finance.yahoo.com/quote/VCEL/?p=VCEL
YOU TUBE VIDEOS, and the fourth video down, you will NOW appreciate the meaning of less invasive on the new FDA approved MACI procedure and why MACI is anticipated to be 20x the procedures of Carticel
https://www.youtube.com/watch?v=y3S1kFfT0Qk
https://www.youtube.com/watch?v=dP5TgnS3Vr4
https://www.youtube.com/watch?v=gCbpFoY6ZUM
https://www.youtube.com/watch?v=3MFL10O-avE MUST WATCH
http://wnyt.com/health/maci-new-procedure-to-repair-damaged-knee-cartilage/4369510/
https://www.dvidshub.net/news/223965/wbamc-employs-state-art-knee-implant-first-time-dod
Conference Calls
September 27, 2016 Ladenburg Thalmann
October 24, 2016 Meeting on the Mesa
November 30th, 2016 The 28th Annual Piper Jaffray Healthcare Conference
December 14th, 2016 Vericel Corporation Investor Call
Investor Relations:
http://investors.aastrom.com/index.cfm
Yummy Land, click on Above Picture
All messages, including iBox content, are the opinion of the posters, are no substitute for your own research, and should not be relied upon for stock trading or any other purpose.
Also, keep in mind that moderators may or may not have a position in said stock. Being a moderator isn't a sign of endorsement.
Please keep your posts on topic because your message(s) will probably be deleted when:
* Posting content that's off-topic to the subject of this board;
* Posting statements that don't add value to the discussion; or
* When you violate any other posting term of the iHub User Agreement: http://investorshub.advfn.com/boards/complex_terms.asp
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
