Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Your charts sure do fire me up sharpei... Thanks again... Looking forward to earnings and the CC this week..!!
ELTP: Fibonacci Targets using the Fibonacci Arc as well as mathematical calculations for confirmation.

Click on image to enlarge.
As we embark on this incredible journey with ELTP, the chart with critical price points becomes more relevant.
As you know, the target of 1.33/1.34 has been noted here on several occasions, but we don't know when we will hit that number, or what the resistance points should be until now...at least that is the hope.
What we do know is that .97 is our most recent all time high; I have pegged .1201 as a bottom point of that rise. If you note, .97 / by .1201 is '8'. Let it be also noted that a calculation of .20 X '8' is 1.60, our target for the Fib #1.62, referred to as the 'golden ratio'.
From .97 (or .9703) I will use Fibonacci #s to target stops along the way to our ultimate (at this time) $2 (100%).
.9703 X 1.08 = 1.05;
.9703 X 1.382 = 1.34;
.9703 X 1.5 = 1.455;
.9703 X 1.618 = 1.57;
.9703 X 2 = 1.9406.
There is no warranty as to the accuracy, real or implied, of this information.
VICL: June 12 Two Years Daily Chart.

Technical Indicators on 6 months chart

Click to enlarge image.
No warranties of accuracy is implied.
Thank you ash111 for the following:
'VICL, more info: HCW starts Vical at buy : June 12, 2015 by leonardzehr
buy4H.C. Wainwright has launched coverage of Vical (NASDAQ:VICL) with a “buy” rating and a target price of $2.50, based on a discounted revenue and earnings valuation methodology. The stock closed at $1.47 on Thursday.
Vical’s core franchise is comprised of a broadly applicable vaccine technology platform and is developing ASP0113 to address cytomegalovirus infections, a serious unmet need in immunosuppressed hematopoietic stem cell transplant and solid organ transplant patients.
“In our view, these two markets combined address a $1.8-billion opportunity and represent low hanging fruit in terms of clinical and regulatory success,” writes analyst Mark Breidenbach.
In addition, Phase 2 data from Vical’s Herpes Simplex Virus vaccine program is expected imminently, he added.
Mr. Breidenbach said that with a newly licensed anti-fungal asset, Astellas as a Big Pharma partner, and a cash position of approximately $46.3-million, which should be sufficient to fund operations into 2017, “we believe Vical represents an under appreciated story with potential upside for long-term, risk-oriented investors.”
biotuesdays.com/2015/06/12/hcw-starts-vical-at-buy/ '
SVON: Thank you for posting the info on SVON, ash. I really do like the chart!!! In fact, it looks FANTASTIC!!!!!!!
SVON For those not familiar with Billionaire Philip Frost,take a look here : http://investorshub.advfn.com/Phillip-Frost-Plays-27154/
The buyers of those $3m shares expects at least a double (probably 3-5x from $.75),imo. Actually he owns a ton from before, just invested more.
Sevion Therapeutics, time to rock n roll, this stock will have a ton of action going forward now that Opko is involved. Check the chart of other stocks he was involved with to see what Dr. Frost can do with small market cap stocks like SVON.
SNS01-T (oncology)-Clinical!, http://www.seviontherapeutics.com/pipelin
13m cap and just raised $3.3m
NEXT WEEK: "Sevion Therapeutics to Present at 2015 BIO International Convention" http://www.seviontherapeutics.com/
SVON also working with Mayo Clinic (like TPIV),"More than 3,300 physicians, scientists and researchers from Mayo Clinic share their expertise to empower you". "Mayo Clinic has major campuses in Rochester, Minn.; Scottsdale and Phoenix, Ariz.; and Jacksonville, Fla. The Mayo Clinic Health System has dozens of locations in several states"...
http://www.mayoclinic.org/
Eagle1, I will try to restrain myself from here on, but this is 06/11 post from Couch:
Couch Member Level Thursday, 06/11/15 08:13:37 PM
Re: None
Post # of 156619
'A few more additional FACTS about the Actavis/Epic/Elite connections that Nasrat is the key to AND additional REASONS I would not want to bet against Nasrat.
1) Notice the highlighted RED portion below. Nasrat has had EXTENSIVE dealings with the FDA while at Actavis. It's why he knows how to deal with the FDA professionally.
Actavis was/is one of the handful of companies that distributed generic oxycodone and a limited supply of Purdue's ADT OxyContin.
http://www.law360.com/articles/436327/purdue-actavis-settle-oxycontin-patent-battle
When Actavis reopened their Little Falls NJ plant in 2009 after closing it down.....notice it called on Nasrat to get it back in tip top shape and re-launch generic Oxycodone.
Quote:
“Through an extensive process, Actavis re-qualified all equipment and utilities for production and packaging – and we re-qualified and revalidated all methods used to release products from our Totowa facilities,” said Nasrat Hakim, Vice President of Quality Compliance and Technical Services for Actavis Inc. “This is a very positive step and took incredible team work. The next step in the process will involve additional interaction with the FDA so that we can continue to introduce products as outlined in the Consent Decree.”
The FDA completed its inspection and approved the release of the first two products as outlined in the Decree: Oxycodone 15 mg and 30 mg tablets. Subsequent inspections, also as outlined in the Decree, will follow.
2) Notice in 2009 who Actavis hired as their Managing Director of the New Jersey Solid Oral Dose Operations. Yep, Elite's VP of Operations ~ Doug Plassche.
Quote:
From 2009 to 2013, Mr. Plassche was employed by Actavis as Managing Director of the New Jersey Solid Oral Dose Operations, overseeing 450 employees and the production of more than 100 products.
3) Now at some point Actavis needed additional help manufacturing its generic oxycodone. Who did it pick you might wonder. YEP: EPIC .....take a look.
http://dailymed.nlm.nih.gov/dailymed/image.cfm?type=img&name=c088a64a-figure-04.jpg&setid=df9313c5-e454-4829-bbb7-2f26d9f85e1e
4) Now recall Epic had a lawsuit with Purdue pending in which Epic was not able to get their own Oxycodone ANDA approved. Notice that Actavis at the time was involved with Purdue legally as well.
http://www.law360.com/articles/411658/purdue-hits-2-generics-makers-with-oxycontin-patent-suits
5) So anybody want to tell me that EPIC isn't a solid player when Actavis comes to them for manufacturing help regarding Oxycodone.
http://ir.elitepharma.com/profiles/investor/ResLibraryView.asp?ResLibraryID=77479&BzID=2258&t=1948&g=939&Nav=0&LangID=1&s=0
6) Now, notice in Elite's PR that Epic has an ADT ER Oxycodone product.
Quote:
"Epic is pleased to have an opportunity to market this important new product and to add to our current pipeline of opioid products that includes a pending application for an abuse-deterrent oxycodone HCl extended release product," said Dr. Ashok Nigalaye, Chief Executive Officer of Epic. "ELI-200 will allow Epic to extend its reach in the anti-abuse pain space, and to position both companies for long-term growth."
7) So now you will have EPIC going to marketing its own ER ADT OXY and Elite's IR ADT OXY at the same time!!! THAT IS HUGE.
8) What I'm curious to know more about and think it would be a solid question to ASK during the CC ~~~ That Epic ADT it will be using.....will that be a hard turtle shell version like Purdue/Actavis has agreed upon.......OR is EPIC going to using a version of Elite's ADT??? AND if so, can you say LICENSING AGREEMENT.'
Hi GMan: How're you doin'? Aren't we in a sweet spot now. Do you need charts on any other stocks? Or questions? Let me know. Let's enjoy this ride...:)
'CORRECTION -- Elite Pharmaceuticals, Inc. to Host Conference Call and Webcast to Discuss Year End 2015 Financial Results on June 16, 2015
Financials for Year Ended March 31, 2015 Will be Released on June 15, 2015
NORTHVALE, N.J., June 11, 2015 (GLOBE NEWSWIRE) -- In the press release issued earlier today by Elite Pharmaceuticals, Inc. (OTCQB:ELTP) with the same headline, please note that the conference call date should be Tuesday, June 16, 2015 and not Tuesday, July 16, 2015 as previously stated in the conference call details. All other information remains the same.'
MMs likely have an opportunity to keep price lower for one or more days.
Conversation opened. 1 unread message.
Skip to content
Using Gmail with screen readers
marcia
Google+ Profile Icon
Search
Gmail
COMPOSE
Labels
Inbox (5,251)
Starred
Important
Sent Mail
Drafts (204)
Circles
[Gmail]/New folder
[Imap]/Sent
[Imap]/Trash (11)
Junk
Personal
reidtarb
Travel
More
CollapseChat
marcia r
Status menu
Idle David Cline
Offline Margarete Bodmer
More
1 of 7,325
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Attachment
Not starred
Attachment
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Not starred
Attachment
Not starred
Collapse all
Expand all
Print all
In new window
Fwd: CORRECTION -- Elite Pharmaceuticals, Inc. to Host Conference Call and Webcast to Discuss Year End 2015
Inbox
x
clearlake.ca <clearlake.ca@gmail.com>
4:51 PM (2 minutes ago)
to me
Sent from my U.S. Cellular® Smartphone
-------- Original message --------
From: "Elite Pharmaceuticals, Inc. "
Date:06/11/2015 3:26 PM (GMT-08:00)
To: Marcia Rutherford
Subject: CORRECTION -- Elite Pharmaceuticals, Inc. to Host Conference Call and Webcast to Discuss Year End 2015
Release #:2258-152347-rl-1175716:
CORRECTION -- Elite Pharmaceuticals, Inc. to Host Conference Call and Webcast to Discuss Year End 2015 Financial Results on June 16, 2015
Financials for Year Ended March 31, 2015 Will be Released on June 15, 2015
NORTHVALE, N.J., June 11, 2015 (GLOBE NEWSWIRE) -- In the press release issued earlier today by Elite Pharmaceuticals, Inc. (OTCQB:ELTP) with the same headline, please note that the conference call date should be Tuesday, June 16, 2015 and not Tuesday, July 16, 2015 as previously stated in the conference call details. All other information remains the same.
CONTACT: For Elite Pharmaceuticals,
Inc.
Dianne Will,
Investor Relations,
518-398-6222
Dianne@elitepharma.com
www.elitepharma.com
Elite Pharmaceuticals, Inc. Logo
My ResponsePass
Click here to Unsubscribe from our email list.
Thursday, June 11, 2015 6:26:20 PM 1175716
Click here to Reply or Forward
2.6 GB (17%) of 15 GB used
Manage
Terms - Privacy
Last account activity: 0 minutes ago
Details
Almost forgot: ELI-200 scheduled for approval by end of this year.
Copied from ELTP board:
'
Bngo Thursday, 06/11/15 02:08:37 PM
Re: kayak_wench post# 156509
Post # of 156595
Elite^Epic Power Partnership!
I believe this partnership is Brilliant!
As a former AstraZeneca Pharmaceutical Sales Specialist for almost 10 years, I helped launch and promoted many $Billion drugs such as: Crestor, Symbicort, and Seroquel.
There were at least a thousand Sales Reps and dozens of District Sales Managers, Regional Sales Managers, Trainers, and Scientific Affairs personnels at these National launch meetings.
Because these were " brand new compounds", they needed to be promoted aggressively to compete with existing blockbusters such a s Lipitor, Adviar, and Cymbalta in the market place already.
It was all about convincing the physicians why they should try the new drugs based on better efficacy or safety data.
With Elite's potential 17 ADT opioid line, I believe there will not be much convincing needed to be done.
These opioids and naltrexone have been promoted for years already with their efficacy and safety data.
It will be a simple presentation or an awareness campaign of our products.
Same "generic life cycle" compounds with existing safety and efficacy data but with an abuse deterrent safety net for physicians when prescribing these opioids.
My points is, by not hiring 10 sales reps at $100,000+ overall package will save the company over $1,000,000.
This is just rough calculation, but imagine if that was 100 reps x$100,000 = $100,000,000+.
What about 1,000 reps x $100,000 = $1,000,000,000+.
What about the salaries of the layers of middle and top sales managers?
Brilliant in the sense that by not going Big with Big Pharma might it be the 100% correct answer if Elite and Epic can do this all by themselves to cut cost and maximize profit is Brilliant for us as investors.
For a start, an awareness campaign consists of low cost mass form of communications: email, mail, or fax to all drug area distributors such as McKesson, to all pharmacies like CVS, to all opioid prescribing physicians and dentists.
Why would any physician put himself/herself at risk and prescribe the old opioid formulations with abuse, diverting potential vs. Elite's new, safer anti abuse formulations.
Their prescribing behavior will change within days!
Brilliant cost cutting strategy to maximize profit! '
s.
ELTP Oh, North, Eagle...and North again. Earnings will be fantastic as they've had two launches isradipine and hydroxyzine which will show income in the first (israd) and second (hydroxyl) qtrs. The CEO is fantastic; he had 30 yrs in the industry in top mgmt. before coming to E. He loaned (unsecured) $1M to the company and he has acquired about 2.7M shares personally; I think that says a lot. Jerry Treppel, the pres was responsible for bringing NH on board two yrs ago. We went from near bk to .97 (March 05 2014)after this and have come down to .20 base. NH brought his line of generics from his former personal company, Mikah. I wish WeeZuhl (a physician) was still posting as his dialog was an excellent source (disgusted with IHUB admins) but he is there behind the scenes. If you public post to him he will very likely answer; and we have No2Koolaid, lazers, JimmyJoe, mts, Couch and others (Check out the stickys on the E board. Yes, Eagle this will go. Tomorrow I will put up the chart with the targets. But, my long term (by DEC) is likely 1.33 (or higher). We are supposed to launch our first ART ELI-200 and the others will come along quickly after that as the tech is the same (2-bead) and FDA will already hold the data....so boom, boom, boom. Earnings tomorrow after the bell. You will have lost precious time likely as the run is in process. There is just volumes of info on the mb. Get in and good luck! S.
Sharpei! Got the ELTP info...Thank You!
Where do you think it is heading?
Eagle1
Re: Eagle1 post# 42
Post # of 70
eagle1: Thought I'd send this by lasers today to add to DD on ELTP...bottoming today:
'
lasers Member Level Thursday, 06/11/15 10:24:29 AM
Re: Money_Tree post# 156455
Post # of 156466
It is just the opposite. The FDA wants the $ELTP efficacy data for pain mgmt to complete its records. The FDA already has data based on the HAL studies, P1/2A equivalence for both ELI216 and ELI200, and knows exactly what to expect. NH has assured the FDA that the ELI's SOPs have only changed for the better providing more containment of the Naltrexone vs body fluids based on the Granted Patents.
The FDA has had the opportunity to check all of $ELTP's manufacturing facilities Operation Logs and has obviously come away convinced. In fact convinced enough that it is not even testing ELI200 ER polymer for the agonist.
In that case the FDA, obviously under considerable pressure from citizen groups, politicians etc. has IMO asked $ELTP to do what can be done to get Commercial product ASAP!!!!
$ELTP ELI200 new manufacturing facilities, along with sales and marketing, all at $ELTP expense, are now set to go 1H2016. There will be no delays.'
Eagle: There is today's TPIV chart for you on SharpeiDeum, also.
Thanks for the TPIV chart! Had a healthy little pull back and I'm looking for the next leg up to be $1.00+
Talk soon!
Eagle1
Do not overlook ELTP either. Over the next weeks and months there will be a significant rise in price. Read the stickys on ELTP board.
Spickandspan: Please see post titled: 'Fro', as there are two stocks on that for you! S.
Thank you, Spicknspan. Happy to see you. I do all stocks...at least offhand can't think of any I wouldn't.
I am loving this as this is the 'not work if you love it' job we hear about.
Get on board ELTP!!!! It is just starting what will be an historic run. Check out the DD I sent Eagle1. Will think about some others for you and will get back to you.
Let me know what you would like. S.
Interesting board, sharp. Do you look at all kinds of stock--microcaps and NASDAQ's?
What are three stocks that you like right now?
TIA
'
lasers Member Level Thursday, 06/11/15 01:35:41 PM
Re: kayak_wench post# 156509
Post # of 156515
kayak $ELTP made the deal with EPIC for several reasons:
(1) $ELTP and EPIC already share confidential technical information as well as trade secrets on $ELTP ELI ADTs manufacturing process.
(2) $ELTP totally respects EPIC proven abilities to function and deliver on ALL aspects of manufacturing, sales, and marketing.
(3) The least cost to $ELTP is with EPIC services and therefore the most benefit to $ELTP Shareholders.
(4) EPIC is totally trustworthy.
(5) EPIC has all of the facilities and manpower to carry out $ELTP 1st stage of operation expansion beyond $ELTP NJ facilities.
There is more but these 5 reasons are key as to why $ELTP has JV partnered with EPIC. '
and lasers knows his stuff!!! (Charts posted daily on the ELTP site.)
Continued profit-making, Eagle1!)
Sounds like a good plan, IB. Not planning, but it does look good.
Thanks Sharpei !!!!
I am looking at $2.83 to enter, if it falls that low !!!
Are you picking up any?
IB_
eagle1: Thought I'd send this by lasers today to add to DD on ELTP...bottoming today:
'
lasers Member Level Thursday, 06/11/15 10:24:29 AM
Re: Money_Tree post# 156455
Post # of 156466
It is just the opposite. The FDA wants the $ELTP efficacy data for pain mgmt to complete its records. The FDA already has data based on the HAL studies, P1/2A equivalence for both ELI216 and ELI200, and knows exactly what to expect. NH has assured the FDA that the ELI's SOPs have only changed for the better providing more containment of the Naltrexone vs body fluids based on the Granted Patents.
The FDA has had the opportunity to check all of $ELTP's manufacturing facilities Operation Logs and has obviously come away convinced. In fact convinced enough that it is not even testing ELI200 ER polymer for the agonist.
In that case the FDA, obviously under considerable pressure from citizen groups, politicians etc. has IMO asked $ELTP to do what can be done to get Commercial product ASAP!!!!
$ELTP ELI200 new manufacturing facilities, along with sales and marketing, all at $ELTP expense, are now set to go 1H2016. There will be no delays.'
Eagle: There is today's TPIV chart for you on SharpeiDeum, also.
Big thumbs up, IB. Will take a look.
'FRO' had a nice little gain today !! Didn't fall low enough to buy in :( lol
Maybe on the next dip ....
IB_
Thank you Coo2002coo; it's good to hear from an old friend. Quite a history we have!!!!! (Let'em guess.)
Hi, Sharpei. It's always great to learn something from you.
Reprinted from a NASDAQ2020 post 06/10/15(loaded with invaluable data):
'***** ELITE PHARMACEUTICAL *****
--The Company’s primary focus is the accelerated development of a complete line of 17 Abuse Resistant Opiods
-- "We have emerged as one of the leaders in the development of abuse deterrent opioids"
Ellie's products possess characteristics that are best-in-class among abuse deterrent formulations(ADF).
--Elite Pharmaceutical executed an exclusive License Agreement with Epic Pharma, to market and distribute in the United States, ELI-200, an undisclosed opioid with sequestered naltrexone capsules, owned by Elite. Epic will have the exclusive right to market ELI-200 and its various dosage forms.
--Dosing for an ELI-200 Phase III clinical study started. This Phase III study is a multi-center, randomized, multiple-dose, blinded, placebo-controlled, parallel group, study to evaluate the efficacy and safety of abuse deterrent ELI-200 for the treatment of adults with moderate to severe pain following surgery. The study will enroll approximately 165 patients at five clinical sites and last 8 weeks.
--FDA filing to follow with expedited review.
--Commercial launch preparation has begun for ELI-200
-- first abuse deterrent opiod ELI-200 will enter an 11+ BILLION dollar market with very limited competition
--CEO bought 11,700,000 shares on the open market in 2014
--3 New Senior Management Appointments: "These additions greatly strengthen our management team in key areas and enhance our internal expertise in product development, regulatory compliance and intellectual property to support the development of our opioid abuse deterrent products" 10/2014
--Human Abuse Liability (HAL) Studies- HAL 1, HAL 2 and HAL 3 POSITIVE for ELI-200 an undisclosed Abuse Deterrent Opiod for pain, HAL required for valuable FDA ADT labeling
--Multi-Billion dollar pipeline of over 40 drugs(20 FDA approved) which includes 17 NDA Abuse Deterrent Formulation(ADF) opiods
--going concerns removed by SEC
--strongest balance sheet ever
--first working capital surplus in 5 years
--9 QUARTERS AVERAGING REVENUE GROWTH PER QUARTER OF 73%.
--$40 MILLION in funds secured from LPC(Lincoln Park Capital) to develop ADT/ART products, enough for 3-4 ADT opiods
--Generic Business Cash Flow Positive minus R&D costs approaching 2 million in revenues per quarter
--Isradipine launched Jan 2015
--Isradipine in studies for Parkinson's desease and Opiod Withdrawl
--Hydroxyzine launch with limited competition April 2015
--Dantrolene next with a 12 million dollar market
--Elites ART is a MODULAR 2 bead system, it can be used on ALL opiods just add a different opiod beads to the naltrexone(antagonist) beads, once first ADT launches the rest will follow in rapid succession
--Successfully completed pivotal bioequivalence studies on both ELI-201(twice day oxy ADT) and ELI-200(undisclosed twice a day)
--Abuse Liability studies for ELI-200 POSITIVE, required for very RARE and VALUABLE Abuse Resistant Labeling
--Elite's goal is to become the leader in the ART/ADT opiod market "no one will stop us" "we are going to market" CEO
--"We have set in motion several initiatives that will have significant impact on Elite's future" CEO
--aggressive schedule with clinical trials, working on *7 ART opiods* concurrently in 2015, FDA agreed to an***expedited*** review for ELI-200
--Nearly Doubled the FDA-DEA-cGMP registered manufacturing space in 2014 for a total of 50 thousand sq/ft for research, development and manufacturing from concept to commercialization
--Over $500,000 invested in faclity expansion in 2014, new encapsulator, tablet press and high shear grandulator, NEW COMMERCIAL FLUID PROCESSOR has 5X more capacity enough for 2+ ADT opiods in house
--space and funds for 3 in house ADT opiod products
--NEW packaging line operational
--Poison Pill 8-A12G filed and a staggerd Board of Directors in place protects Elite and shareholders from a hostle take over
-- Fall 2013 an independent analyst determined Elite is undervalued and its true value is between $2.10 and $2.75 Elite is currently trading on its generic business NOT as an R&D co. developing multi-billion dollar ADT opiods
--Multiple potential partners approaching ELTP are awaiting trial results. CEO wants to license ART "after studies and trials completed" making it worth much more due to the steep accretion curve in the pharma field ie. the value goes up exponentially as studies are completed
--the longer Elite goes without partnering or buyout the higher the value of the company, no buyout expected prior to first ADF approval
--Nasrat said they have employed a firm to get them Sar-Box compliant. True, this would be important to get to the Nasdaq. But, it would also be important for their being acquired.
--ELI-216 ADT will be the only 24 hour oxycodone in the USA
--ELI-154 CR 24hr (once a day Oxy) in scale up for Large European Market
--20 FDA approved 10 launched and gaining market share: hydromorphone, phentermine 37.5 mg, lodrane D, methadone, phendimetazine, phentermine 15mg, phentermine 30mg, naltrexone 50mg and Isradipine 2.5 and 5.0 mg Hydroxyzine
--Elite has signed a Manufacturing and License Agreement with Epic Pharma who will manufacture 11 of the 12 approved generics ANDAs recently obtained from MIKAH. This will allow Elite to maximize their profit potential for their generic business while devoting their resources to the development of their abuse resistant products. Isradipine manufactured in house by Elite and only has 1 competitor. EPIC filed CBE-30s for additional MAKAH generics
--Elite's Partner Camargo is a full-service drug development partner specializing in the 505(b)(2) process — an approach for developing products that offer differentiated benefits. Camargo is capable of managing every facet of the plan throughout the development continuum, from feasibility assessments, formulation and testing the drug product, to conducting preclinical and clinical studies, to final submission
trial #1 mega pilot 4 way cross over on 64 subjects SUCCESSFUL on 12 hr Oxy/Nal ELI-201
trial #2 and 3 pivotal BE studies SUCCESSFUL for both ELI-201 (12hr oxy) and ELI-200 (12hr undisclosed) ADT
trial #4 and #5 Started 05-19 for ELI-202: the first dosing of a pivotal bioequivalence study in healthy volunteers for ELI-202, an undisclosed opioid abuse deterrent product, utilizing Elite's proprietary pharmacological abuse deterrent technology. Two bioequivalence studies will be run together for ELI-202.
2014 Lifetree 3 tier abuse deterrent studies to obtain * FDA ADT LABELING *
a) snorting trial completed POSITIVE
b) oral abuse POSITIVE
c) IV abuse POSITIVE
Human Abuse Liability study completed to test abuse potential of crushed ELI-200 taken intranasally -POSITIVE
ELI-200 (undisclosed) believed to be a 6 hr oxy is also on schedule for filing in 2015
--Phase III for ELI-200 trial started :
https://www.clinicaltrials.gov/ct2/show/NCT02391571?term=eli-200&rank=1
--pediatric study to run concurrently during approval process
http://seekingalpha.com/article/2859876-elite-pharmaceuticals-is-well-positioned-to-address-7b-abuse-deterrent-opioid-market
http://www.prnewswire.com/news-releases/global-pain-therapy-moderate-to-severe-market-2015-report-on-management-of-pain-288713661.html
http://www.wsw.com/webcast/rrshq24/register.aspx?conf=rrshq24&page=eltp&url=http%3A//www.wsw.com/webcast/rrshq24/eltp/
http://seekingalpha.com/article/1688112-elite-pharmaceuticals-call-it-a-comeback-story?source=yahoo
http://seekingalpha.com/article/1905051-elite-pharmaceuticals-swing for-the-fences
http://seekingalpha.com/instablog/4199131-couch/1941422-elite-pharmaceuticals-eltp-intellectual-property-ip-and-the-right-to-devise-a-better-pain-killer
--FDA likes the pharmalogical approach to abuse resistance FDA very positive
--the ART is rock solid, innovative, unique, superior and CEO has not seen a better tech
--Elites tech is much better at sequestering Naltrexone.
--1st patent for 2 bead ART 8/182,836 abuse resistant oral formulations and method of use thereof (approved May 22, 2012) method for making an abuse resistant drug
--2nd patent for 2 bead ART 8/425.933 formerly 12/640,344 (04/23/2013) combined with first patent, gives Elite 20 years of propietary protection: formulation to make an existing drug abuse resistant
--3rd ART patent 8,703,186
--4th Hammerlock Patent 13/379,481 microtablets for use with Elite's abuse resistant products June 2015
--Patents good thru 2023
--**Canadian Patent Number 2,521,655 titled "Abuse-Resistant Oral Dosage Forms and Method of Use Thereof”. Issuance 03/2014 expands the scope and reach of Elite’s patent estate internationally. Elite has additional patents pending in the U.S., Canada and Europe.
--Additional European and Canadian Patents Pending
--Elite may license these ART products at a later date to a third party who could provide funding for the remaining clinical studies and who could provide sales and distribution for the product.
--Patent pending 13/379,486 microtablets 0.25-1.0mm, smallest in the industry for use with ALL medicines
--patent 12/075,816 for Sequestering polymer acrylic co-polymer composition: ON HOLD while Elite and lawyers Woodcock & Baker strategize to add more claims
***The recent guidances and actions by the FDA related to extended release opioids and specifically oxycodone demonstrates the FDA support for abuse resistant technologies
Opioid products incorporating abuse resistant technologies have become a public health priority and these recent actions appear to be a major step towards the FDA eventually requiring that all extended release opioids utilize these technologies.
January 9, 2013, the FDA released its new draft guidance document, titled "Guidance for Industry: Abuse-Deterrent Opioids - Evaluation and Labeling". The document discusses what it will take for an opioid formulation to secure an abuse resistant label. The implications for companies that meet these expectations are significant and even more so for those that meet these requirements and have patented abuse resistant technologies.
March 2013, 48 state attorney generals called on the agency to require generic makers to produce tamper-resistant versions of opioid medicines
--the STOPP ACT - a bill before Congress NOW, introduced by US Rep's Rahall, Rogers and Keating. The law will prohibit any old-formula opiod from entering the market if the FDA has an equivalent abuse deterrent/resistant opiod approved
--CLADD petitions FDA to reject all opiods without ART www.cladd.org
--FDA's Purdue ruling April 16, 2013 : prohibits new original non-abuse resistant oxycodone from being made in generic form, unless it has an abuse deterrent/resistant properties. FDA will not accept or approve any ANDA applications based on the original OxyContin formulation
--the FDA decision basically requires generic companies to buy or develop their own abuse deterrent/resistant technology: it effectively eliminates much of Elite's competition which has no ART thus making Elite's 2-bead ART MORE VALUABLE
--going foward all new generic and NDA branded opiods will be required to have Abuse deterrent/resistant technology
--2-bead abuse resistant opiods will gradually replace non-abuse deterrent opiods now on the market. FDA wants to replace non-ADF opiods with safer ones
--CEO and officers being paid with stock
--multiple partners Watson/Actavis, Mikah, Epic, TAGI, The Pharm Network, ECR, Precision Dose, Celgene Corp, TPN/Ascend, Novartis, SmithGlaxoKlein and the undisclosed Hong Kong Pharma
--contracting deals with other pharmas
--40+ employees over double of two years ago
--phentermine 15 & 30mg launched (April 11, 2013) is competing with Qsymia(phentermine and topiramate)
--Acend Labs has new contracts for methadone
--HITK's intermediate for a generic of a branded 100 million dollar market= drug Lots produced
--Lodrane 24D equivalent waiting on feed back from FDA they will tell them which pseudoephedrine salt to use and which product to use to run trials with
--undisclosed 100% Elite owned ANDA
--Elite's goal is to commercialize a COMPLETE LINE of 17 abuse resistant opiods
--Uplisting to the NASDAQ exchange per the CEO
--ELTP visibility increasing: Once the pilot and pivotal studies completed CEO to go on the road to present the results to viable partners
--Nasarat Hakim former VP of Activas appointed president and CEO of Elite 08-05-13(pps $0.068) has 30 years of pharmaceutical and medical industry experience in Quality Assurance, Analytical Research and Development, Technical Services and Regulatory Compliance. He brings with him proven management experience, in-depth knowledge of manufacturing systems, development knowledge in immediate and extended release formulations and extensive regulatory experience of GMP and FDA regulations. He is making ART priority one. He bought 11 million shares on the open market and takes ELTP stock as compensation. The CEO noted, we are trading on our financials(revenues) and if we had no revenue we would be trading on ART and R&D and we would be valued ***10X*** higher
NORTHVALE, N.J., Feb. 17, 2015 (GLOBE NEWSWIRE) -- Elite Pharmaceuticals, Inc. ("Elite" or the "Company") (OTCQB:ELTP) announced guidance today regarding the Company's Phase III trial for ELI-200, an abuse deterrent opioid product. Elite has submitted a study protocol to the FDA and dosing for the study is expected to begin shortly after the FDA comments on the protocol are received by Elite. The Company anticipates that it will receive the FDA comments by mid-March and that dosing will take approximately eight weeks to complete.'
Thank you, Naz!
Big thanks, Couch!!! Welcome to the site for everyone, every stock, but most of all 'our little engine'!
Congrats on getting this site up and running Sharpei. Couldn't happen to a nicer person. I've appreciated all the solid TA re: ELTP.
Best as always,
Couch
Thanks G. The Little Engine just keeps on chugging.
IMO we continue up into the CC next Tuesday... As we will undoubtedly get an earnings report Friday or Monday right... Then they will probably sell the news.. Keep up the good work.. Love the new site..
Thanks, GMan: I am working diligently to figure out where we go from here. Do we start a typical slow steady slide like we usually do; after all if that tall candle is a Wave 5 (and we need to at least come close to that .27), and we do not keep up the momentum we might experience something like this: a push up a bit to resistance and a pull back to the 50 DMA. ?? , I think this is my greatest challenge for now. Just hang in and hang on cause it IS going HIGHER. S.
Excellent post sharpei..!!
I think there will be a better place to get in..Will get back to you after I see the move tomorrow, which I am expecting will rise slightly and reverse. Unless there is more news in the am, which is unlike the company to do so.
1)'
I would take nothing off the table (except BK and RS). You raise an interesting point. Some institution could, indeed, come in and buy the company at a value that must be close to some reasonable estimate of the valuation. Remember, they would have to make the price satisfactory to shareholders so they would forestall any threat of litigation.
So, let's play with some math again...if we take the average of 40 cents, 2.10 & 2.75 that gives us a p/s of 1.75. That would be a steal for the institutional investors, yet shareholders would likely be very pleased (if we factor in the time value of money). Best guess is the institutional investors would hold it and let it grow for a bit, then sell it at a premium closer to what we long term shareholders believe is a reasonable, longer term price. Just a thought.'
2)Perhaps you were not aware of previous posts I have made about the topic. But, before addressing the question of value, I want to make it clear that I have stated on a number of occasions (as a review of my posts would reveal) that it is common for firms to pay a 30-40% premium, as any DD would discover. With that, let's do the math...and, since it is my math, I get to make the assumptions.
Remember the valuation completed on Elite? We all know the range included a low of 40 cents and a high of 2.75, with an interesting mid-point of 2.10 (which we would agree is not the median, but I digress). What was the basis for that valuation? Answer: the existing business at that time (November 2013). So, fewer patents, fewer products, fewer revenues, as yet to be completed trials, smaller manufacturing capabilities and, of course, no product at the doorstep of an abbreviated P-3. With all of that, even the basement valuation identified a projected p/s that exceeded the p/s at that time - which was 12 cents. So, we need to be clear that a valuation would more fully appreciate that Elite's true value extends beyond a current p/s.
Since it is common knowledge that a valuation would exceed the p/s based on specific catalysts as reflected with the aforementioned accomplishments, we can assume Elite is worth more now than they were at the time of the previous valuation. How much more? Ah, that is the question. If we look at the success rate for firms having completed a P-2, we know they are 25% more likely to receive an approval. But, we also know firms that begin a P-3 are 60% likely to receive approval and for those having completed a P-3 the success rate for approvals is 90%. With this in mind and the increased number or patents, products, revenues, successful trials and expanded infrastructure including manufacturing, what might be the increased value of Elite? Well, I believe they are at least 40% more valuable. So, now we can put the numbers together and everyone on the board can argue to agree or disagree. But, the logic is sound and unless and until someone offers another version that is grounded in reality (and two cents is not reality), this is what I believe...
Throwing out the 40 cents which Elite argued was a "scorched earth" p/s (but played much differently), I will assume a better point for use is the mid-point between 2.10 and 2.75. I suggest 2.50 because it makes the math a bit easier to follow. With 2.50 times an assumed increase value of 40%, our math is...2.50 X 1.40 = 3.50 p/s. To this we need to assume a reasonable premium, so taking the midpoint 30-40% premium range, that means...3.50 X 1.35 = 4.73 p/s. Or about ten cents below what someone had previously suggested. If we take a midpoint between the current O/S (611 Million) and what I have projected to be the number of O/S at the time I expect an offer to be made (725 Million), that is 668 Million shares outstanding times 4.73 and that gives us a projected offer of...$3.16 Billion. Actually lower than I have projected.
Agree or disagree, it does not matter. I have offered simple and reasonable assumptions. Now, if I wanted to be unreasonable or was some egregious pumper, I would argue a much higher number based on a higher value. But, that requires assumptions I do not need to make. So, that is my story and I am sticking to it...
3) I Hope I am not posting the same over and over N2Koolaid, I believe is a former corporate officer of more than one large corporation. There are several extremely well-qualified people on related subjects: WeeZuhl is, I believe an emergency room physician, and of course lasers, who is well known across small bios, I think has run labs...great source and strong supporter.
Thanks, Eagle1....
Thank You Sharpei!
Will check this ELTP and other one you mentioned!
Stay in touch and have a good evening!
Eagle1
Thank you, Eagle. We'll chat some more later. Congrats. Are you in ELTP? It had a nice run (for a beginning) this am and earnings, CC and other good news coming this month!
Hi Sharpei, TPIV
I appreciate your charts and TA, but this one seems to be a lot different as the FA, along with a Billionaire long (DART cups) as a heavy investor too believing in this company and not selling.
Golden Cross forming? Let's keep a close eye on this one....
Talk soon and have a great evening!
'Elite Pharmaceuticals and Epic Pharma Enter Into a Sales and Distribution Licensing Agreement for Abuse-Deterrent ELI-200
NORTHVALE, N.J., June 9, 2015 (GLOBE NEWSWIRE)
http://globenewswire.com/news-release/2015/06/09/743155/10137768/en/Elite-Pharmaceuticals-and-Epic-Pharma-Enter-Into-a-Sales-and-Distribution-Licensing-Agreement-for-Abuse-Deterrent-ELI-200.html
-- Elite Pharmaceuticals, Inc. ("Elite" or the "Company") (OTCQB:ELTP) announced today that it has entered into a sales and distribution licensing agreement with Epic Pharma LLC for ELI-200 (the "License"), an abuse-deterrent opioid utilizing Elite's proprietary pharmacological abuse-deterrent technology. Epic will be responsible for sales and marketing of ELI-200, and Elite will be responsible for the manufacture of the product. Epic will pay Elite non-refundable milestone payments totaling $15 million and a royalty based on net product sales. The term of the License is five years and the License is renewable upon mutual agreement at the end of the initial term.
Elite also announced today the dosing of the first subjects for an ELI-200 Phase III clinical study. This Phase III study is a multi-center, randomized, multiple-dose, blinded, placebo-controlled, parallel group, study to evaluate the efficacy and safety of abuse deterrent ELI-200 for the treatment of adults with moderate to severe pain following surgery. The study will enroll approximately 165 patients at five clinical sites.
"Epic is pleased to have an opportunity to market this important new product and to add to our current pipeline of opioid products that includes a pending application for an abuse-deterrent oxycodone HCl extended release product," said Dr. Ashok Nigalaye, Chief Executive Officer of Epic. "ELI-200 will allow Epic to extend its reach in the anti-abuse pain space, and to position both companies for long-term growth."
"I am delighted to have a partner like Epic for Elite's first abuse-deterrent product," said Nasrat Hakim, President and Chief Executive Officer of Elite Pharmaceuticals. "We will work closely with Epic to prepare for and ensure a successful launch. The ELI-200 Phase III study is expected to be wrapped up later this year and we expect an NDA filing for ELI-200 by year's end."
Additional information about the licensing agreement and Phase III clinical trial will be provided at our yearend conference call following the filing of our annual report on form 10-K for the fiscal year ended March 31, 2015. We anticipate that the conference call will be held on June 16th, 2015. Additional information about the conference call will be provided.
http://globenewswire.com/news-release/2015/06/09/743155/10137768/en/Elite-Pharmaceuticals-and-Epic-Pharma-Enter-Into-a-Sales-and-Distribution-Licensing-Agreement-for-Abuse-Deterrent-ELI-200.html'
It should be a quieter day on the board with the naysayers hiding under a rock.
I deserved that Eagle1. GLARING error! Let that be a lesson to me as I begin this public journey. I hope you will allow me to make up for that, but if not I do wish you success always. My motto: I break it; I own it. BTW, did you see out little ELTP at long last begin its (hopefully second huge triumphant win since March 2014?)
Congratulations on TPIV 'American Pharoah' finish.

Thank You Sharpei!
Eagle1
IB: It looks to me that you could have a small trade at the 2.6 but if you wait I believe it looks like it could hit 2.5ish. An extreme drop it could do about 2.43.
Sharpei Diem!...You, too can learn to determine price direction without having to struggle through numerous online studies. |

'Keep It Simple Stupid' in the Complicated World of Stock Trading and Investment.
Thinking about where to best get in or out of your targeted stock? When you doubt where to put in a 'buy' order, go for the lower price even if means waiting -and you are chomping at the bit . Fibonacci numbers are most helpful as are technical indicators: moving averages, long term as well as short term chart assessments, Elliott Wave, rsi, macd, slow stochastics, adx/dmi, Donchian Channels, etc. in conjunction with Fundamentals to include the latest Q's, paying particular care to net assets.
(When considering small caps, I tend to pay particular attention to and avoid those that show reserves out of balance when compared to future requirements to operate, low floats, possible bk or 'lawsuits' mentioned on message boards and heavy finger-pointing at management found on message boards. (This last can be unreliable if there is an unusually high complement of postings of questionable or malicious intent.)
| Some believe technical analysis works, while others feel it's akin to reading tea leaves. Judging by the feedback comments on this column, reader opinions are sharply divided too. The fundamental analysts who didn't see the fall in the oil price, or who told us that gold was going back to $1,500, or that the DOW would collapse didn't seem to attract as much trenchant criticism as those of us involved in technical analysis. In 2014 we produced weekly CNBC blogs providing trading outlooks based on technical and chart analysis. We use chart analysis to establish the probability of trend change and to set price targets and objectives. In 88 percent of analysis notes in 2014 the price targets were achieved or exceeded. That's around the same percentage of correct calls in 2013 and 2012. The analysis methods we use are not complex; they can be applied by anyone without the need for a Master's degree in finance. These methods are covered in my books, including Guppy Trading. Charting analysis provides both the calculated price targets and the price levels that indicate the trade has failed. In 12 percent of cases, the analysis is not correct, but chart analysis provides exact price levels that signal this decision in real time. 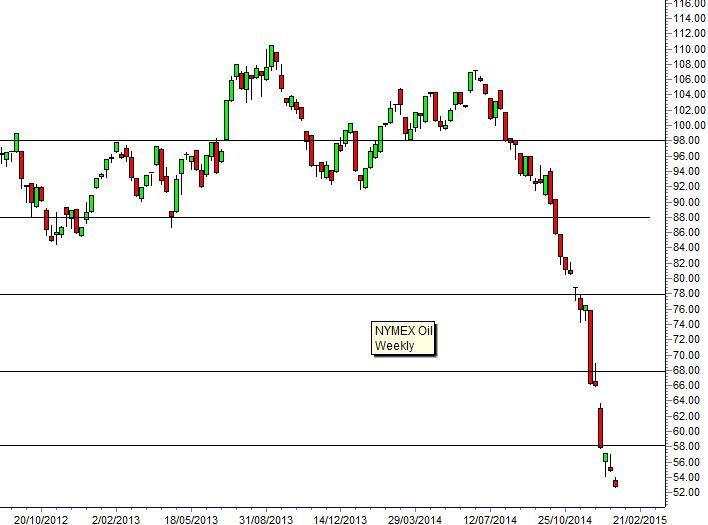 Our best calls for 2014 included the January 2013 DOW target of 17,000, NASDAQ at 4600 and S&P at 2000. They also included oil at $88, and then later $68 and $58. The next downside support target for NYMEX oil is $48. Gold support near $1,180 and the Shanghai Index upside targets above 3000 were also other correct analysis calls. Our worst call was the NIFTY where we expected the parabolic trend to develop into a 50 percent or greater retracement. There was a correction, but it was not a major change in the trend. Two bullish calls on the Australian dollar were also incorrect, with the market failing to break through resistance levels. Chart analysis is designed to identify situations where high probability outcomes are expected. Often chart analysis can be used to set exact price targets. The CNBC notes apply the same analysis methods we use in our personal trading and we use them because they work. Daryl Guppy Contributor This site is not intended to be a full course of study as there would be no time to process members' charts and questions, but there will be an attempt to encapsulate the oft-asked critical issues with TA as they apply to most of you. For more in depth study I have noted above under 'Author's Sources' and highlighted text quality sites that can be accessed online. (If you have a particular question regarding the action of an indicator or related to this process, just post it and I will answer to the best of my ability. Thanks for your patience with the limitations of this site. Using Technical Indicators The following is a short cut to finding an entry point but if you use most or all these 'tests' before jumping in, you should have some protection from overpaying a stock. (It is imperative that you use these in conjunction with the following time charts: Weekly one year, two year, five year, twenty (not all necessarily but use about three); AND. also do the same with the daily _ and even take a look at a couple of monthly charts (one to twenty years depending on how long its been listed, of course); then the final critical data to cement your decision should come from very short term - from six months daily to 60, 30, 10, 05 mins (in multiple time frames - two to 20 days, for example). Moving Averages: 50 DMA... Does this support a current price or are you looking forward to resistance at this MA? Same with 200 DMA. It's a happy chart that shows an upward cross over the 200 DMA by the 50. Use these in conjunction with the other Tech Indicators.
***Notice: I would like to welcome aboard one other chartist, Conix from the IHUB family of contributors. He, too will be adding his expertise in this area to those who wish help in their investment choices and approaches. Rules of Investing 1-10 are Keep It Simple Stupid X 10.  |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
