Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Regenicin had some tough times with former partners as we all no, things are very very well now , there is no need to worry about 2 million in debt for a company like this , in matter of no time things will get better for those who have been patient, this is a long term stock and will pay off for all of us , Regenicin has no employees as of right now, once this 10 million comes I'm sure Randy will rehire the crew back , as of right now board members friends and family kept this life saving technology alive , because everyone believes in Randy , board , and FDA never seen anything like this miracle, not to mention there are 5 other patients that go along with the skin , everyone that's been patient will get a big rewarded $$$$$$$$$$$$$$$$$$$$$$$$$$$ Money and IND approval coming very very quickly, don't miss out buying this stock you and the next few generations will be set.
RGIN being over $2,000,000 in the hole and only $12,000 cash on hand McCoy is going to need that $10,000,000 to get things righted.
Who is Randy's crew? The board members or does RGIN have employee's?
There are 35 IND approvals in the FDA office as we speak and Regenicin is one of them so funding is a pice of cake once the news come out , automatically people with big money will jump to fund the project all the way through, even though they have a 10 million deal on the table depending if Randy and the board except it , they have to be very careful to structure the deal because as we all no lots of wolves out there . Believe me we all will thank Randy and the crew ,
Randy all along said this is a life saving technology, there is no such thing like this even the FDA believes very strongly we all no good things take a long time now the bubble is going to burst IND news coming out very shortly along with the funding , you think Rany would let his friends,family and all the investors down , Randy thanks for all the hard work you and the board have done so far .
Thats is wonderful news Nicole1129, by chance, what is your source for this information?
There is about 10 million in funding coming up shortly, also IND news at the same time , this is going to be a hit $$$$$$
Anyone have an idea on status of IND or other RGIN important points of progress...this is getting old fast...
Grtherd...thanks for reply. Any thoughts on status of RGIN IND process/project w/FDA?
Guys there is big big news coming out soon , this stock is going in the range of $3 to 8 dollars a share , load up people, there is big $$$$$$$ in funding and IND info jackpot jackpot jackpot
Hi Randy,
A pathogen-free herd is an impossibility, maintaining a "Closed Herd" can be a smart move for a small farm. It can decrease the need for testing frequency and reduce the risk of introducing diseases into your herd. However, Canada is a better possibility. Manitoba is a better option than New Zealand. We in Canada would welcome your investment. There are areas in the boreal forest where there are no pathogens. The permafrost is melting so there is hope for pathogen free herds. However, we in Canada are in need of the latest biomedical news about the USA. The USA is on the forefront of biomedical research and we in Canada are enamored of your research.
I'm talking about north of Toronto, north of Winnepig, north of Montreal...north of Vancouver...
Keeping a fully closed herd requires a good deal of effort on the part of the farmer.
It goes well beyond simply breeding your own replacement livestock, and rather embraces awareness around disease exposure. A fully closed herd means that livestock don't have any contact with saliva, feces, urine, blood, semen, etc. A farmer who values a <<CLOSED HERD>> must keep in mind all of those potential paths for infection.
On some farms the level of biosecurity may not be possible or practical. In that case a higher frequency testing may be warranted to verify herd health (in New Zealand..ha ha it's going to cost you money to import the beef parts). For other farmers who value a closed herd the effort may be well worth it to avoid the frustration that comes from dealing with a disease on a small farm. On some farms this level of biosecurity may not be possible or practical. In this case a higher testing frequency may be warranted to maintain herd health. How are you going to ensure the health of the herd when you are living in New Jersey, Randy, and the herd is in New Zealand? For other farmers though, the effort may well be worth it, to avoid the frustration that comes from dealing from disease on a small farm.
1) Screen all Livestock before bringing them into your farm.
2) In addition to screening new livestock, purchase only from sellers who also maintain proper screening
3) Screen all cattle and goats, including livestock kept for meat
4) Do not share bulls or bullocks with other farms.
5) Reduce contact with wildlife with fencing, and/or the use of guardian
5) Do not board or pasture livestock from other farms.
6) Be mindful of contact with neighbour livestock, across the fence.
7) If you purchase new animals, keep them isolated for at least 2 weeks to make sure that they are not incubating a disease. If an animal becomes sick in isolation in the pen or stall, you have an opportunity to clean that area, removing all feces, and using an appropriate dis-infectant. Rather than using the sick animal infecting your herd.
8) Some diseases (like BVD "bovine disease diarreah") can be devastating. One infected animal brought to your farm can be disastrous, even if your cattle are vaccinated against this disease, because vaccinations are never 100% effective.
Keeping a "FULLY CLOSED HERD" is an impossibility, because livestock disease can pass
through saliva, mucuous, feces, urine, semen, blood and en utuero.
9) New Zealand cattle are also prey to deer, elk, etc. This herd of yours is not going to be sacrosanct nor "Pure" as you would imagine.
10) The Maori people are tired of being used by people from the West.
Hello out there,
There are a lot of closed herds in North America, one is called "California Herdshare Association". Then there is the Tractor Supply Ad. Closed herds are not unique to North America nor are they unique to New Zealand. There is also the https://www.ag.ndsu/livestockextension/ranch-hand-newsletter/is-your-herd-really-closed
Then again you could ask the Texas Agricultural Extension Service to do an experiment with closed herds. You don't need to go all the way to New Zealand to have a closed herd where you can garner and cultivate the tissue samples. It's not necessary to travel to New Zealand for a closed herd. You can have a closed herd in Texas, in Maryland, anywhere. Just my humble opinion.
Sarah.
PS...there is a pathogen-free pig farm in Darlington, MD. Archer Farms, Inc. with 140 purebred pathogen-free herd of pigs from Yorkshire females.
Yes,
I called one time...I think I spoke w/him directly. He was encouraging me to keep expecting news...that was months ago
I really cant say since I dont know how long it takes to isolate a herd to purify their collagen for development. Last I knew McCoy was going to announce something in December 2016. I havent been following them that closely. McCoy was is New Zealand sometime over the summer but since then I havent kept up. Its anyones guess at this point. Have investors been calling the company???
Scott...any guess on timing? It will not take much to get this going.
I havent paid attention since I sold but farming Collagen takes time so until his herd is heard, RGIN is the penny slots at the Casino
Clocks ticking.,...... Lack of input and info will crush this to negative value. RGIN would be well advised to release updates.
Sold the .055's when RGIN hit .13, looks like that was a good trade as RGIN hasnt done much since then.
I heard whispers of McCoy being in New Zealand to check on his herd. Does anyone know of any development. The December 2016 deadline is long past due.
I might be a buyer again in the .02's for a little bump when Randy has more than Golden Corral reviews (the man can EAT)
Is RGIN static b/c we await new FDA guidelines for trials? New trial rules, technical, other will slow things up. Just a thought, held up like everyone else till sometime in Sept.
They already have orphan drug designation.Funding for phase2 study is the issue. Silence from company mean they don't have funding.
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm565148.htm
This new initiative may help speed things up a little.
We sure are due.
Skin...appreciate the new information. I would normally be unaware of NZ TV. If you have other relevant RGIN news pls pass it along. I do own a lot of this and hope it works out for all, including the patients who benefit.
I am surprised that no one picked up on this. Randy was in New Zealand to discuss closed-herd farming. There was even an interview on Maori TV on May 28th. Keep the faith. Things are moving.
I am and remain a believer. Just need calibration on timing, according to my clock we are soon getting into Q3 which is later than I expected based on milestones and other plans.
This will turn and run up shortly. Needs time and proper guidance. Which it's getting.
Nothing on specific direction or data dump. Ya just gotta hold on. Multiple times I've said this is long term. Any market is always on term for long haul. Day trading and quick turns are nonsense and provide no stability. If it tanks and fails you've not lost much at penny stock value. When it hits and takes off. My handle says it all. Risk = Reward.
Apple was a supposed dump until things went in another direction in 2004; as was GenZyme earlier than that. The list goes on........ Perspective and accouabilty on McCoy exists. Keep in mind it's a sma ll show that has limited resources. His info is very public. You will get a response if your concerned. Again my view long term. Let it run. If it fails . You didn't drop 110.00 a share on AIG or Sherson Lehman that you loose everything you own on someone's whim.
Pay attention. Ask questions. Get out if not comfortable.
Apple...hope you are right. I have confidence in the team but some days it is tough. Can you say at milestones in particular that you expect to see action on?
I liked the way they were laid out and the emphasis.
Things will be looking up soon.. Milestones to be accomplished.
No idea, 400 shares traded isnt a sign good news is coming. McCoy said he'd have developmental news in hand by December, its JUNE. The volume will indicate somethings in his wheelhouse. Until an announcement is made with something positive RGIN is pretty much dead money. Watch for a increase in Volume, its a sure sign of positive development.
Anyone know why this thing poped 40% today? Been waiting a long time to see it move. Hope this means they have FDA action or funding support...green is good
When is this stinker going to grow some legs?
Still holding above 12.
Don't let those sneaky market makers steal shares from you. Who is dumping at .095 cents? Really No news out and just a vacumn. Guys panic and market makers get cheap shares. Stay long, they are accumulating shares, then will run it up in shorts or guys who got out's face.
If there is a big bid in level 2, they will sell to get someone to sell along with them so they can be filled at their price. Look what happened once .095 traded. Next trade .1055 then up to .1075, then 11, 11.5 13.5, .14 then .15 (last few on low volume, but low was 50% LESS).
Again.. sshhh. This is long haul. There is no quick return or fast gain. Buy it sell it or hold it.
Just buy it and hold it. It may take a while. But my user name says it all. Hold long. This one has steam, purpose and utility.
Anyone have ANY news?!? WAY TOO QUIET!
Ssshhhh. Everybody's busy working... Pass it on.
aggressive buyer this morning - news out soon
Surprisingly no uptick on the positive positioning and guidance for 2017?
Clearly a decent amount of progress
Gotta stop flipping and let this one ride a bit
Regenicin Reports 2016 Operating Results, Outlines Milestones for 2017
PR Newswire PR NewswireJanuary 18, 2017Comment
LITTLE FALLS, N.J., Jan. 18, 2017 /PRNewswire/ -- Regenicin, Inc. (OTC Bulletin Board: RGIN), a biotechnology company specializing in the development and commercialization of regenerative cell therapies to restore the health of damaged tissues and organs, recently reported the Company's operating results for 2016 and an outline of key milestones for 2017.
As the Company described in its recent 10-K filing with the SEC, 2016 has been a year of substantial accomplishments. The Company's contract laboratory completed its evaluation of NovaDerm®, a regenerative cell therapy that has the potential to re-grow a patient's own skin in a laboratory by harvesting a small, stamp-size skin biopsy. The results proved that NovaDerm® can be successfully grown in the laboratory on the Company's proprietary collagen scaffold. The final research report was a crucial part of Regenicin's FDA submissions for an Orphan Designation and Pre-IND (Investigational New Drug) meeting with the FDA.
The Company completed a contract with Bovine Collagen Products (BCP) to manufacture its proprietary collagen scaffolds, the platform on which skin cells are grown to produce NovaDerm®. The Company also completed a contract with Pure Med Farma, LLC (PMF) which will supply closed herd collagen to produce the proprietary scaffold. The use of closed herd collagen is now a requirement outlined in FDA guidance documents to ensure animal source materials are safe for use in humans.
In August, Regenicin secured an Orphan Designation, as a biologic, from the Office of Orphan Products Development. The FDA's Orphan designation is granted to promote the development of unmet clinical needs with new therapies for rare diseases and disorders. Once approved as an Orphan Product, NovaDerm® would be granted 7 years of market exclusivity by the FDA. In addition, there are other financial benefits along with the FDA's support during the approval process.
In September, Regenicin submitted a pre-IND meeting request and met telephonically with FDA officials at the end of October 2016. The Agency addressed Regenicin's questions and provided guidance on the Company's planned NovaDerm® development pathway going forward. It was agreed that PMF would perform the testing and specifications as per the American Society for Testing and Materials to fulfill current ASTM F2212-11 requirements. Regenicin is working closely with PMF to ensure this testing can be completed promptly.
And finally, Regenicin selected a well-known and respected global Clinical Research Organization (CRO) that will assist with the final Investigational New Drug (IND) application submission and the clinical trial process, including site selection, documentation, physician training, patient recruitment and a final clinical report on the results of the trials. An IND application is the document filed with the FDA to request permission to proceed with clinical trials. This application includes the candidate product's research and development, chemistry manufacturing controls and processes, pre-clinical safety, related product studies, clinical trial protocols, and development history.
Milestones for 2017
The primary initiative for the first quarter of 2017 is to raise additional funds to finalize the data needed for submitting the IND application. It is estimated that the cost to complete the IND will be approximately $1.5 million, and the cost to complete the Phase I clinical trial will be approximately $2 million. The main focus in obtaining this funding is to minimize shareholders' dilution as much as possible. Consequently, Regenicin is primarily pursuing financing through the issuance of a debt instrument, as well as international licensing agreements.
Regenicin has begun the preliminary planning for the clinical trials to the extent the Company is able based on funding constraints. As mentioned above, Regenicin has chosen a CRO to assist in the IND submission and conducting the trials. Clinical site selection and patient recruitment should be faster than normally expected, as the Company is only scheduling 10 subjects at two burn centers.
In Summary, Regenicin intends to achieve the following milestones in 2017:
Secure interim financing to finalize product development and testing needed to support the IND
Complete a second round of financing in order to conduct 10 subject clinical trial with NovaDerm®
Work with Pure Med Farma to finalize Collagen Scaffold Testing and Specifications for NovaDerm®
Finalize contracts with the CRO and select the NovaDerm® manufacturer
Retain a Principal lead investigator, medical advisor, surgical trainer and dermopathologist
Select and execute contracts with two clinical study sites
Enroll first subject into the trial
Perform successful grafts of NovaDerm onto clinical trial subjects
About Regenicin
Regenicin, Inc. (OTC Bulletin Board: RGIN), is a biotechnology company specializing in the development of regenerative cell therapies to restore the health of damaged tissues and organs. Regenicin, which was founded in 2010, has assembled a world-class management team with a proven track record for developing and bringing innovative medical devices and biotechnology products to market. The company is publicly traded with headquarters in New Jersey. For more information on Regenicin, Inc., as well as its technologies and products, please visit the company website at www.regenicin.com.
Here are other companies in 10K, either bought out or much bigger market caps, so there is definitely a big interest in this market. Raising debt for $2-4M should not be hard.
Mkt Cap
Amarantus Biosciences, Inc. 1.7M
Smith & Nephew Wound Management 14.5B
Genzyme Biosurgery bought Out
Integra Life Sciences Corporation 3.2B
LifeCell Corporation/Kinetic Concepts KCI bought Life for 1.7B in 2009, sold in 2016 to Alergan at 2.6B
Organogenesis Inc private but have 2 products 240 employees, new product announced Oct 2016
Intercytex liquidated and became private in 2010
Genzyme bought out
Advanced Biohealing/ Shire huge 50B, busted for kickbacks on their dermaplast
Cy Ttera/ NovoCell/ViaCyte all merged partners with Perkins Elmer, mostly diabetes
Biomimetic Therapeutics Inc. bought by wright medical 2.5B
RTI Biologics 200M
With RGIN at 20M Market Cap, still cheap.
Interesting. Upturn and stable hold.
|
Followers
|
71
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
2935
|
|
Created
|
08/06/10
|
Type
|
Free
|
| Moderators | |||
Regenicin, Inc.
10 High Court
Little Falls, NJ 07424
**updated and correct phone number** (973) 557-8914
they filed this on aug 22 2016- http://www.otcmarkets.com/edgar/GetFilingHtml?FilingID=11558959
| Name: | Randall McCoy |
| Title: | Principal Executive Officer and Director |
| Date: | August 22, 2016 |
| Name: | John J. Weber |
| Title: | Principal Financial Officer and Director |
| Date: | August 22, 2016 |


LITTLE FALLS, N.J., June 12, 2012 /PRNewswire/ -- Regenicin, Inc. (OTC Bulletin Board: RGIN) a biotechnology company specializing in the development of and commercialization of regenerative cell therapies to restore the health of damaged tissues and organs, announced today that the Food and Drug Administration (FDA) has granted Orphan Status approval for the PermaDerm® product, the only tissue-engineered skin prepared from autologous (patient's own) skin cells consisting of both epidermal and dermal layers, that is indicated for catastrophic burn patients. The FDA had previously designated PermaDerm® to be a biological/drug (permanent skin replacement) not a medical device (temporary skin replacement). PermaDerm® is the only skin replacement technology to receive this Biological/drug designation.
COMPANY HIGHLIGHTS:
PermaDerm™ is being developed to be the only tissue-engineered skin prepared from autologous (patient's own) skin cells consisting of both epidermal and dermal layers. A small harvested section of the patient's own skin can be grown to graft an area one hundred times its size in as little as thirty days.
These living, self-to-self skin graft tissue are intended to form permanent skin tissue that will not be rejected by the immune system of the patient, a critical possibility in porcine or cadaver skin grafts used today.
The technology has been clinically tested in over 150 pediatric, catastrophic burn patients. Currently Regenicin is working with its contract manufacturer to prepare for pre-market approval of PermaDerm™ from the FDA.
PermaDerm™ is being designed to save lives, reduce healthcare costs by decreasing the patient's stay in the Critical Care Unit and reduce the need for additional surgeries. An insurance company procedural code has been approved for reimbursement of costs to hospitals. The American Medical Association has assigned CPT (Current Procedural Terminology) code for cultured skin substitutes under the dermal substitute category which enables insurance companies to process and hospitals to be reimbursed for cultured skin substitutes once approved by the FDA.
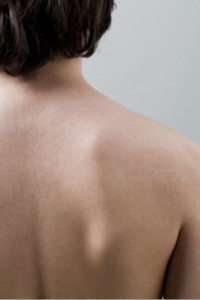
According to the American Burn Association, there are currently over 2,000 cases annually involving burns covering over 50% of the patient's total body surface area.
PermaDerm™ is being developed for the treatment of catastrophic, full-thickness burns covering over 50% of the total body surface area.
PermaDerm™ has been used in clinical trials on pediatric patients with catastrophic burns covering from 50 to 90+% of their body.
PermaDerm™ is being developed to require less donor skin from the patient's own body, thus needing fewer surgeries to harvest skin. The result is expected to be less scarring, with fewer infections and faster healing.
Randall McCoy
Chief Executive Officer
Randall McCoy has more than 37 years of experience in the healthcare industry. His experience includes executive positions and scientific research and development. His previous employment includes George Washington University, Temple University Medical School and RCA/SRI International (Director of Life Sciences and Electronic Displays). Mr. McCoy has assisted both small and major pharmaceutical/device companies address FDA issues. He has also helped over 225 foreign and domestic companies introduce their FDA regulated drug and medical device products into the US and World market. Mr. McCoy currently has over 30 Patents US and International.
Changed to AMBS
Dr. Joseph Rubinfeld Ph.D
Director at CytRX; Initial Co-Founder of Amgen, Inc.
Dr. Joseph Rubinfeld co-founded SuperGen, Inc. in 1991 and has served as its Chief Executive Officer and President, Chief Scientific Officer and as a director. He has been a director of CytRX since July 2002. Dr. Rubinfeld is also a founder of JJ Pharma. Dr. Rubinfeld was one of the four initial founders of Amgen, Inc. in 1980 and served as a Vice President and its Chief of Operations until 1983. From 1987 until 1990, Dr. Rubinfeld was a Senior Director at Cetus Corporation and from 1968 to 1980, Dr. Rubinfeld was employed at Bristol-Myers Company. Dr. Rubinfeld received a B.S. degree in chemistry from C.C.N.Y. and an M.A. and Ph.D in chemistry from Columbia University.
John Weber
Regenicin™'s Interim CFO
John Weber most recently served as Executive Vice President of Fujifilm USA, the highest ranked American corporate officer, from 2006 until his departure in 2009. His responsibilities included overseeing all corporate activity with the exception of R&D. During that time Fujifilm was ranked as the fastest growing medical imaging company, consistently ranking #1 or #2 in customer satisfaction. From 1998 through 2006 he served as Senior Vice President, Operations at Fujifilm USA where he spearheaded the transition of the company from a film distributor to a digital medical informatics company. From 1986 until 1998 he served as CFO where he helped to profitably manage Fuji's growth from an employee base of 75 to over 1,000. Prior to his distinguished career at Fujifilm USA, Mr. Weber served as the CFO for the confectionary and drinks division of Cadbury Schweppes Limited for three years and as Corporate Controller for an additional five years.
Dr. Craig Eagle
Pfizer Oncology
Dr. Craig Eagle joined Pfizer Australia in 2001 as part of the medical group. In Australia, his role involved leading and participating in scientific research, regulatory and pricing & re-imbursement negotiations for compounds in therapeutic areas including oncology, anti-infectives, respiratory, arthritis and pain management. In 2003, Pfizer relocated Dr. Eagle to the United States where he was appointed as the worldwide lead for development of Celecoxib in oncology to oversee the global research program. Since that time he has had increasing responsibility for overseeing the global research plans and teams for Irinotecan and Dalteparin. In 2007, he became head of Medical Affairs and Outcomes Research for Pfizer, including the US oncology business. Dr. Eagle has led, or been directly involved with, teams that resulted in eight new products or indications. As part of his current role at Pfizer, he has led the integration of the Pfizer/Wyeth oncology businesses and portfolio.
The Broadsmoore Group, LLC 9,223,770(8)
560 Lexington Ave., 16 th Fl.
New York, NY 10022
PDA Associates LLC 7,770,000(9)
560 Lexington Ave., 16 th Fl.
New York, NY 10022
Officers, directors and 5 percent shareholders collectively 54,508,099(10) 59.74%
* Less than 1%
(1) Unless otherwise indicated, each person or entity named in the table has sole voting power and investment power (or shares that power with that person's spouse) with respect to all shares of common stock listed as owned by that person or entity.
(2) A total of 83,807,964 shares of the Company's common stock are considered to be outstanding pursuant to Rule 13d-3(d)(1) under the Securities Exchange Act of 1934.
(3) Includes 32,821,641 shares of common stock held in his name and options to purchase 885,672 shares of common stock.
(4) Includes 50,000 shares of common stock held in his name and options to purchase 885,672 shares of common stock,
(5) Includes 1,050,000 shares of common stock held in his name and options to purchase 885,672 shares of common stock
(6) Includes 50,000 shares of common stock held in his name and options to purchase 885,672 shares of common stock,
(7) Includes 33,971,641 shares of common stock, options to purchase 3,542,688 shares of common stock and shares of Series A Convertible Preferred Stock convertible into 3,350,000 shares of common stock,
(8) Includes 5,706,270 shares of common stock held in its name, warrants to purchase 167,500 shares of common stock and shares of Series A Convertible Preferred Stock convertible into 3,350,000 shares of common stock,
(9) Includes 7,400,000 shares of common stock held in its name and warrants to purchase 370,000 shares of common stock,
(10) Includes 47,077,911 shares of common stock, warrants to purchase 537,500 shares of common stock, options to purchase 3,542,688 shares of common stock and shares of Series A Convertible Preferred Stock convertible into 3,350,000 shares of common stock
http://ih.advfn.com/p.php?pid=nmona&article=5144097
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
