Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
$PDSB
$PDSB management team is living the dream with shareholders like this person.
— Adam Feuerstein ✡️ (@adamfeuerstein) March 27, 2024
Please, take more of my money!!!! pic.twitter.com/Y3eKD50H2L
PDSB January 19, 2024
Call options: $7.50 Open Int. 1094 $10 Open Int. 2257
Put options: $5.00 Open Int. 873 $7.50 Open Int. 984
Implied volatility is over 100%
PDSB excellent setup for next leg up... Very bullish for it
Ticker Buzz Cloud
24 Hours
12 Hours
6 Hours
3 Hours
1 Hour
AABB
AAPL
AGYP
AMC
AMZN
ANY
APSI
BIEI
BIEL
BIG
BJDX
BLUE
CVSI
DBMM
DFCO
ENZC
ERBB
FOR
GMVD
GOLD
GVSI
HAS
HHSE
HIRU
IFUS
IGEX
JPM
KBLB
KEGS
LWLG
MONI
NBIO
NEXT
NWBO
OPEN
PBHG
PCTL
PDD
PFE
PHIL
RDGL
REAL
RVNC
SNPW
TSLA
WHEN
WHY
WNFT
XCRT
Lol crypto not looking there right now too bearish.... Pharmaceuticals where the money is and oil for the summer run up
Need to look again...I haven't looked so nice the weekly stochastic hit 90 plus last week
Thoughts on efxusdt? Looks like inverse head and shoulders with divergence on stochastic
Got your pm ...Stochastic 90s on the weekly correction time...
Maybe...The weekly stochastic is over 90 though same with other my other biotech plays ...
Can it run more??
https://ycharts.com/companies/PDSB/news
.. 12/28 ..PDS Biotech Reports Median Overall Survival (OS) of 21 Months in Advanced, Refractory Cancer Patients Having Few Remaining Treatment Options and with Reported Historical Survival of 3-4 months
Source: GlobeNewswire Inc.
PDS Biotechnology Corporation (Nasdaq: PDSB), a clinical-stage immunotherapy company developing a growing pipeline of targeted immunotherapies for cancer and infectious disease, today announced expanded interim data in a Phase 2 clinical trial investigating the PDS0101-based triple combination therapy in advanced human papillomavirus (HPV)-positive cancers. The triple combination of PDS0101 with the tumor-targeting IL-12 fusion protein M9241 (formerly known as NHS-IL12), and bintrafusp alfa, a bifunctional fusion protein targeting two independent immunosuppressive pathways (PD-L1 and TGF-ß), is being studied in CPI-naïve and CPI-refractory patients with advanced HPV-positive anal, cervical, head and neck, vaginal, and vulvar cancers.
The triple combination Phase 2 trial (NCT04287868) is being conducted at the Center for Cancer Research (CCR) at the National Cancer Institute (NCI), one of the Institutes of the National Institutes of Health.
All patients in the study had failed prior treatment with chemotherapy and 90% had failed radiation treatment. The interim efficacy data (n=50) involves 37 HPV16-positive evaluable patients, including 29 patients who have, in addition, failed treatment with CPIs (CPI refractory). Highlights of the expanded interim data are as follows and are consistent with the results presented at American Society of Clinical Oncology (ASCO) Annual Meeting 2022 and prior interim data announced in October:
Median OS is 21 months in 29 checkpoint inhibitor refractory patients who received the triple combination. The reported historical median OS in patients with CPI refractory disease is 3-4 months.
In CPI naïve subjects, 75% remain alive at a median follow-up of 27 months. As a result, median OS has not yet been reached. Historically median OS for similar patients with platinum experienced CPI naïve disease is 7-11 months.
Objective response rate (ORR) in CPI refractory patients who received the optimal dose of the triple combination is 63% (5/8). In current approaches ORR is reported to be less than 10%.
ORR in CPI naïve patients with the triple combination is 88%. In current approaches ORR is reported to be less than 25% with FDA-approved CPIs in HPV-associated cancers.
Safety data have not changed since October’s update. 48% (24/50) of patients experienced Grade 3 (moderate) treatment-related adverse events (AEs), and 4% (2/50) of patients experienced Grade 4 (severe) AEs, compared with approximately 70% of patients receiving the combination of CPIs and chemotherapy reporting Grade 3 and higher treatment-related AEs.
“The expanded data continue to demonstrate the durability and tolerability of the PDS0101-based triple combination therapy in advanced HPV-positive cancers, an extremely challenging population of refractory and previously untreatable HPV-positive patients,” stated Dr. Frank Bedu-Addo, President and Chief Executive Officer of PDS Biotech. “We are pleased to see the continued consistency in the data with each update and we look forward to meeting with the FDA to discuss the registrational pathway.”
Both M9241 and bintrafusp alfa are owned by Merck KGaA, Darmstadt, Germany, and its affiliates.
About PDS Biotechnology
PDS Biotech is a clinical-stage immunotherapy company developing a growing pipeline of targeted cancer and infectious disease immunotherapies based on our proprietary Versamune® and Infectimune™ T cell-activating technology platforms. We believe our targeted Versamune® based candidates have the potential to overcome the limitations of current immunotherapy by inducing large quantities of high-quality, potent polyfunctional tumor specific CD4+ helper and CD8+ killer T cells. To date, our lead Versamune® clinical candidate, PDS0101, has demonstrated the potential to reduce tumors and stabilize disease in combination with approved and investigational therapeutics in patients with a broad range of HPV-positive cancers in multiple Phase 2 clinical trials. Our Infectimune™ based vaccines have also demonstrated the potential to induce not only robust and durable neutralizing antibody responses, but also powerful T cell responses, including long-lasting memory T cell responses in pre-clinical studies to date. To learn more, please visit www.pdsbiotech.com or follow us on Twitter at @PDSBiotech.
About PDS0101
PDS Biotech’s lead candidate, PDS0101, combines the utility of the Versamune® platform with targeted antigens in HPV-positive cancers. In partnership with Merck & Co., PDS Biotech is evaluating a combination of PDS0101 and KEYTRUDA® in a Phase 2 study in first-line treatment of recurrent or metastatic head and neck cancer, and also in second line treatment of recurrent or metastatic head and neck cancer in patients who have failed prior checkpoint inhibitor therapy. A Phase 2 clinical study is also being conducted in both second- and third-line treatment of multiple advanced HPV-positive cancers in partnership with the National Cancer Institute (NCI). A third phase 2 clinical trial in first line treatment of locally advanced cervical cancer is being performed with The University of Texas, MD Anderson Cancer Center.
KEYTRUDA® is a registered trademark of Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Biotech mover
overbought on the stochastic too many lower lows on the chart
Just hot a intraday head n shoulders....consolidation
Not on a Friday
Gonna hit the 10's today
weeeeeeeeee weeeeeeeeeeeee
Yup very explosive
Weeee clear to 11s
PDSB Update:
PDSB the price per share is $7.27
+$1.67 (29.82%)
Volume
11,876,752
---------------------------------------------
I got in at 6.93, after alerting here.
------------------------------------------------
https://stockcharts.com/h-sc/ui?s=PDSB
------------------------------------------------------
BRIEF-Pds Biotech Announces 100% Clinical Response In Cervical Cancer Patients In Mid-stage Study
By Reuters — 7:51 AM ET 11/14/22
Nov 14 (Reuters) - Pds Biotechnology Corp (PDSB.NaE):
* PDS BIOTECH ANNOUNCES 100% CLINICAL RESPONSE IN CERVICAL CANCER PATIENTS IN PRELIMINARY DATA FROM IMMUNOCERV PHASE 2 CLINICAL TRIAL
* 89% (8/9) HAD NO EVIDENCE OF DISEASE (COMPLETE RESPONSE) ON DAY 170.
\---------------------------------------
PDS Biotechnology Q3 EPS $(0.26) Beats $(0.28) Estimate. Cash Balance Of Approximately $71.6M
By Benzinga — 7:32 AM ET 11/14/22
PDS Biotechnology (NASDAQ:PDSB) reported quarterly losses of $(0.26) per share which beat the analyst consensus estimate of $(0.28) by 7.14 percent. This is a 8.33 percent decrease over losses of $(0.24) per share from the same period last year.
PDSB PDS BIOTECHNOLOGY CORP
$6.87 +1.2701 (+22.68%)
https://stockcharts.com/h-sc/ui?s=PDSB
BRIEF-Pds Biotech Announces 100% Clinical Response In Cervical Cancer Patients In Mid-stage Study
By Reuters — 7:51 AM ET 11/14/22
Nov 14 (Reuters) - Pds Biotechnology Corp (PDSB.NaE):
* PDS BIOTECH ANNOUNCES 100% CLINICAL RESPONSE IN CERVICAL CANCER PATIENTS IN PRELIMINARY DATA FROM IMMUNOCERV PHASE 2 CLINICAL TRIAL
* 89% (8/9) HAD NO EVIDENCE OF DISEASE (COMPLETE RESPONSE) ON DAY 170
0
What really stands out from PR this morning;
PDS0101 for the Potential Treatment of HPV16-Positive Head and Neck Cancer
In the final KOL presentation, Dr. Jared Weiss provided a review of the ongoing Phase 2 clinical trial (VERSATILE-002) investigating PDS0101 in combination with Merck’s KEYTRUDA® (pembrolizumab) in patients with HPV16-positive recurrent and/or metastatic head and neck cancer. Dr. Weiss discussed the design of the study and data observed from 17 patients, including an objective response rate of 41.5%, clinical benefit rate of 76.5%, and an overall survival rate of 87.2% at nine months. Published results indicate the overall response rate of KEYTRUDA® alone as 19%, as noted in published reports. Dr. Jared Weiss also discussed the lack of differences in poor clinical outcomes for HPV-positive and negative patients with recurrent or metastatic disease.
https://finance.yahoo.com/news/pds-biotech-announces-clinical-trial-130000756.html
GLOBE NEWSWIRE) -- PDS Biotechnology Corporation (Nasdaq: PDSB), a clinical-stage immunotherapy company developing novel cancer therapies and infectious disease vaccines based on the Company’s proprietary Versamune® and Infectimune™ T-cell activating technologies, today announced the initiation of an Investigator-Initiated Trial (ITT), MC200710, for PDS0101 alone or in combination with the checkpoint inhibitor, KEYTRUDA®, in patients with HPV-associated oropharyngeal cancer (HPV(+)OPSCC) at high risk of recurrence. The trial is being led by Drs. David Routman, Katharine Price, Kathryn Van Abel, and Ashish Chintakuntlawar of Mayo Clinic, a nationally and internationally recognized center of excellence for the treatment of head and neck cancers.
Sorry for the lengthy post but I believe this is critical to my investment in PDS and it’s complicated but worth your time. Some of what is discussed below are facts that have been presented by the company or NCI followed by my interpretation/guess as to what it means. After you read this, you will understand why I am so excited about PDS potential, and why I believe the street is missing something incredible and why it is my largest investment.
In June 2021 we learned that 16 out of 18 HPV16++ patients were still alive at 8 months, an incredible outcome in such a difficult to treat group. This included 10/12 CPI refractory patients who are among the sickest patient population with a life expectancy of 3-4 months with current standard of care. The other 6 patients were CPI Naïve patients with a life expectancy of 7-11 months using current SoC and all of whom were still alive at 8 months. The NCI treated 7 HPV16 negative patients who did not meet the criteria for objective response, so they decided to stop treating this group. In summary, in June 2021 they reported on a total of 25 patients who were treated with the combo, 18 who are the targeted population and 7 who are not.
Last week we learned that among 37 evaluable patients (more on this below) the median survival was >12-months at 12/31/21. This is incredible when you look at the overall survival in current standard of care in this population group. Yesterday I received confirmation that the 37 patients included the 7 “off-target” HPV16 negative patients. Median means that half the patients are above a particular number and half are below. That would mean that median survival means half the patients (19) in this population survived for more than 12 months, and the other half (18) have not YET survived for at least 12 months.
At this point we don’t have the details that will come later in the year, but I believe we can infer some very important information. Here is where my speculation and interpretation of the data comes in. Please let me know if I am missing anything or not interpreting the data correctly.
The 37 evaluable patients include patients who have been treated with the combo therapy AND who have received the necessary scans to be considered evaluable, not necessarily that they were in the trial for more than 12 months at 12/31/21. This means it includes patients who were treated in 2020 AND patients who were first enrolled and treated in 2021. This is an important point to remember. Some of those who were treated and considered evaluable haven’t been in the trial for 12 months at 12/31/21 and still 19 out of 37 evaluable patients survived for more than 12 months. Think about how incredible that is but it gets even better.
When the full dataset comes out and we see the details, I believe that the >12-month survival milestone will be achieved by way more than 50% of those treated. I come to this conclusion based on the 37 patients included as evaluable. We know 7 evaluable patients were HPV16 negative. Those patients didn’t have Objective Responses to the treatment based on ASCO data and new enrollment was stopped as a result. I think since they didn’t respond we can unfortunately assume most of them did not achieve the 12-month survival milestone. This would leave 30 targeted patients included in the 37 evaluable. Remember, some of the 30 remaining were considered evaluable because they received treatment and had required scans but were first treated during 2021.
How many were first treated during 2021 and therefore couldn’t be included in the >12-month survival median? We know from ASCO that there were 18 on target patients treated for 8 months as of May 2021, meaning they were treated before 10/2020, 8 months before data cutoff for the presentation. We also know 16 out of these 18 were alive for at least 8 months and counting per ASCO.
There are 12 patients remaining in the target group (30 minus 18) that we don’t know when they were first treated. Even if we assume 1 per month for 12 months beginning 10/2020 forward, it means there were 3 patients treated in 2020 with the remaining 9 treated in 2021. If this assumption is close, that would mean that out of the 37 evaluable patients, 9 were treated in 2021 leaving 28 patients who were in the trial prior to 12/31/20.
We know 19 patients survived for more than 12 months per the HC Wainwright update where the median survival was greater than 12 months. If 19 out of 28 treated in 2020 survived for more than 12 months and 7 of the 28 were HPV16 negative, how incredible is that? Now assume most, if not all the HPV negative patients did not survive for >12 months, it would mean close to 19 out of 21 targeted patients achieved the >12-month survival milestone.
We know from the trial design that there will be 36 CPI refractory patients and 20 CPI naïve patients in the trial. The life expectancy of the refractory population is 3-4 months, and the life expectance of the naïve patients is 7-11 months. What is a reasonable assumption for the blended life expectancy of the treated group? Since there are more patients in the refractory population, using a blended average life expectancy of 5-6 months to compare to the median of > 12-months (and possibly growing) shows just how incredible the results are. This is what the street is missing.
This trial is in a very hard to treat population with a very high unmet need. When this trial completes and the data are available, how long will it take for the NCI to have meetings with the FDA for accelerated approval based on the study assuming the full data are as good as what we have seen to date? What would be a reasonable assumption for approval and more importantly, revenue coming into PDS? How is this trading at a market cap of less than a billion right now? What am I missing?
PDS0203 combines the utility of the Versamune® platform with a recombinant native Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) protein recognizable by our immune system (antigen). The Versamune® platform, due to its unique ability to induce both antibody and T-cell responses is being utilized to develop a vaccine that may more effectively prevent COVID-19.
In January of 2020 the world saw the emergence of the COVID-19 outbreak caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) declared COVID-19 a global health emergency in January of 2020. Within three months of initial discovery COVID-19 was declared a global pandemic, reflecting alarming levels of spread and severity and resulting in unprecedented action by local and national governments to restrict the movement of citizens to contain the spread. Building immunity to the disease is the key to stopping its spread. A COVID-19 vaccine would train the immune system to recognize and destroy the virus without the vaccinated person getting sick.
Coronaviruses mostly infect animals, including birds and mammals. In humans, they mostly cause mild respiratory infections. However, some recent human coronavirus infections have resulted in lethal endemics, which include the Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) endemics. Reported studies suggest that SARS-CoV-2 is similar to SARS-CoV based on analysis of the full-length genome. Various studies of SARS-CoV infection suggest a protective role of both antibody and T-cell immune responses. Multiple reports suggest that antibodies generated against SARS-CoV, were highly prevalent in SARS-CoV-infected patients. Although effective, the antibody response was determined to be short-lived in convalescent SARS-CoV patients. In contrast, T-cell responses have been shown to provide long-term protection more than ten years after infection. PDS Biotech is utilizing the unique immunological properties of Versamune® to develop a transformative vaccine that induces both antibodies and T-cells against the virus.
PDS0203 is currently in pre-clinical development.
PDSB...$12.53...Psar flipped Bullish as the MACD Crosses Positive...
Back in again ...Hedging bids at the Open Gap as well...
Chart... https://schrts.co/cUsjSfVE ...
800% gain on PDSB is my "personal green deal", very thankfull for that and watch the train to pickup on Downside Valley. NOT UN Organisation NEW WORLD ORDER CREATED throw billion of billions from taxpayers. Its time to make BIG RESET OF CHAZARIANS, THE NULL SOLUTION.
5 countb9n the ELLIOTT WAVE
Weird sell off today. Oh well, nice adds. Onward and upward
Certainly is now, so just added
What a ride. Can't believe I sold half my position during the stall around $11...
BREAKOUT!
Thats the 2nd time we had a 15.99 close. i still want to see it close over 16.00 RSI at 66 that could be a good launchpad
Compared to the blood bath the rest of the markets had today I think PDS held its ground pretty well.
With some really good news too. We move forward ![]()
Holding well here. Would be great to see some data soon or an update from Brazil
$PDSB
09/09/2021 PDS Biotech Completes Enrollment of Lead-In Safety Cohort in VERSATILE-002 Phase 2 Combination Trial of PDS0101-KEYTRUDA® in Recurrent or Metastatic Head and Neck Cancer
https://finance.yahoo.com/news/pds-biotech-completes-enrollment-lead-123000620.html
The churn out says blue skys.....I hope we stay 15s till next week for the kaboooom....
|
Followers
|
47
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
1585
|
|
Created
|
06/17/20
|
Type
|
Free
|
| Moderators | |||
https://pdsbiotech.com/images/pdf/presentation/2021/march/PDSB_Corporate_Overview_-_March_11_2021_FINAL_1.pdf  |
DEVELOPING POWERFUL, SAFE, VERSATILE IMMUNOTHERAPIES |
 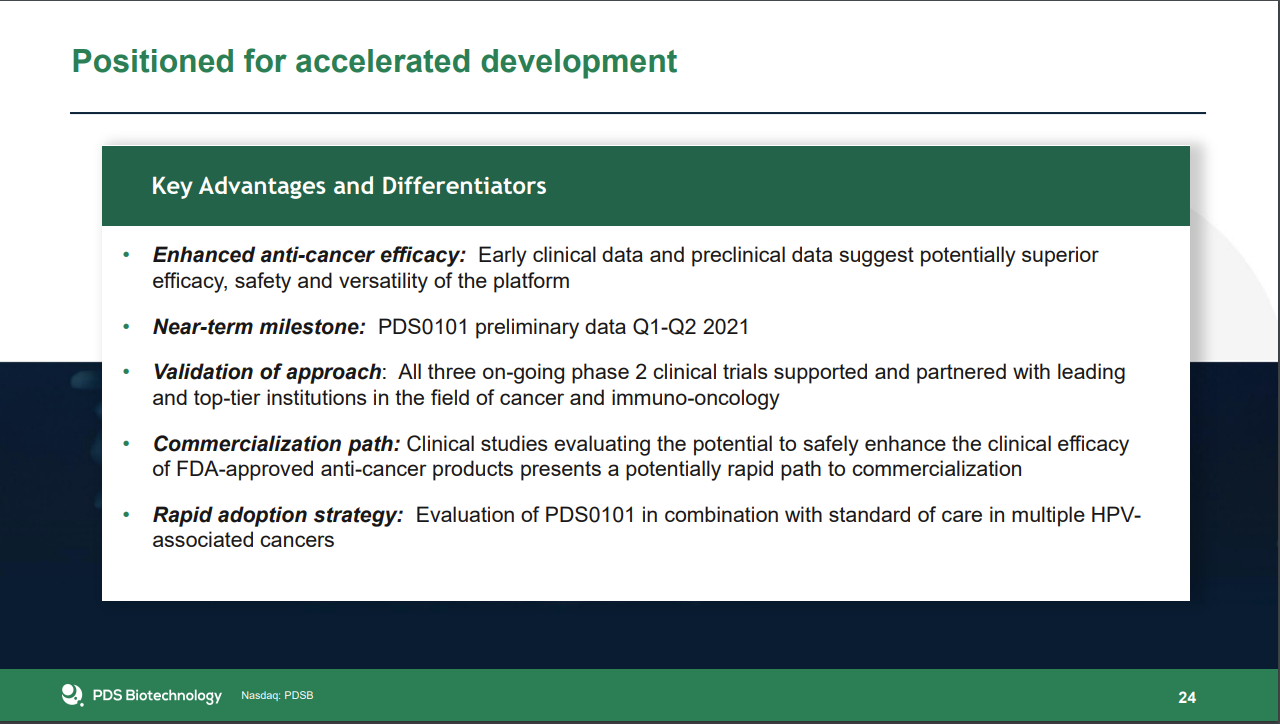 |
| PDSB Versamune® T cell Platform. Our Versamune® platform trains the immune system to unleash a powerful targeted T-cell attack and could dramatically improve treatment and patient outcomes across the cancer spectrum. |
PDSB analyst coverage: |
SECOND GENERATION COVID-19 VACCINE PHASE 3 Q4 2021 PDSB $100.00 PRICE PER SHARE =2.2 BILLION MARKET CAP |
| PDSB biz presentation 2021: PDSB White Paper: https://pdsbiotech.com/images/pdf/presentation/2021/Whitepaper-2021_v6.pdf PDSB Biotech preparing to initiate 3 CANCER Phase 2 clinical studies for lead candidate PDS0101: https://www.youtube.com/watch?v=iYZRmRmRtUI&t=2s OPPENHEIMER Annual Healthcare Conference MARCH 16TH 2021 https://wsw.com/webcast/oppenheimer9/register.aspx?conf=oppenheimer9&page=pdsb&url=https://wsw.com/webcast/oppenheimer9/pdsb/2708189 PDSB website: |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
