Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Can someone feel in this gap? Since NVCN is selling north of $29, yet it's a done deal at $27.25. WHY???
4 to 5 years ago trading was at $119 even after a R/S, why did Boston Sci., and Edwards Life Sci., among dozens of others turn there noises up.
Acceptance is only a couple months away with the FDA.
How can we be sold down the river?
FREE NVCN REPORT
27.96
6.42 (29.81%) Upgrade to Real-Time
Regular Market
Shockwave Medical Announces Agreement to Acquire Neovasc
January 17 2023 - 08:00AM
GlobeNewswire Inc.
Print
Share On Facebook
Shockwave Medical, Inc. (NASDAQ: SWAV), a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely calcified cardiovascular disease, today announced it has entered into a definitive agreement to acquire Neovasc Inc. (NASDAQ,TSX:NVCN), a company focused on the minimally invasive treatment of refractory angina.
The Neovasc Reducer System is a first-of-its-kind technology to address refractory angina. Refractory angina is a chronic condition in which a patient suffers chest pain that cannot be controlled by conventional therapies. It is estimated that each year, in the U.S. and the E.U. alone, up to 300,000 new patients with obstructive coronary disease who are ineligible for conventional revascularization experience refractory angina, despite guideline-directed medical therapy. In addition, it is estimated that up to another 500,000 new patients present with angina and non-obstructive coronary artery disease (ANOCA) in the U.S. and the E.U. each year. The Neovasc Reducer System has been granted Breakthrough Device designation by the FDA, is CE-marked and is currently enrolling patients in the COSIRA-II study, a randomized clinical trial being conducted under an Investigation Device Exemption intended to support U.S. FDA approval for patients with coronary obstructive refractory angina.
“Refractory angina is a debilitating condition without an effective therapy that impacts millions of patients,” said Gregg W. Stone, MD, FACC, MSCAI, Principal Investigator of COSIRA-II, Director of Academic Affairs for the Mount Sinai Heart Health System, and Professor of Medicine (Cardiology) and of Population Health Sciences and Policy. “The ongoing COSIRA-II randomized trial has been designed to definitively demonstrate that the Reducer is superior to a sham control for these patients, offering the potential to change the lives of these patients who are desperate for a solution for their refractory angina.”
“Our team at Shockwave has proven that we excel at developing products and markets for large, underserved patient populations and commercializing innovative solutions for these patients. We believe the Reducer is an excellent fit for Shockwave as it enables us to apply our capabilities to address another large, unmet need within cardiology – refractory angina,” said Doug Godshall, President and Chief Executive Officer of Shockwave. “The timing is ideal as there will be no distraction to our U.S. sales organization in the near term and, as we did with C2, our coronary device, we expect to refine our commercialization approach and begin the development of international markets in advance of U.S. approval. This transaction supports our commitment to drive growth through innovation and we are excited for the potential to bring even more solutions to our customers and the patients they serve with the Reducer System.”
Terms of the Neovasc Agreement
Upon the closing of the transaction, Shockwave Medical will acquire all outstanding Neovasc shares for an upfront cash payment of $27.25 per share, corresponding to an enterprise value of approximately $100 million, inclusive of certain deal-related costs. Neovasc shareholders will also receive a potential deferred payment in the form of a non-tradable contingent value right (CVR) entitling the holder to receive up to an additional $12 per share in cash if certain regulatory milestones are achieved. The upfront cash consideration represents a premium of 27% and 68% to the closing price and 30-day VWAP, respectively, of Neovasc’s common shares on the Nasdaq Capital Market on January 13, 2023.
The transaction will be effected by way of a court-approved plan of arrangement pursuant to the Canada Business Corporations Act, and is subject to customary closing conditions, including requisite Neovasc shareholder approval. Shockwave expects to complete the transaction in the first half of 2023.
The Board of Directors of Neovasc, acting on the unanimous recommendation of a special committee comprised of independent directors and after having received an opinion from its financial advisor to the effect that the consideration to be received by Neovasc shareholders pursuant to the plan of arrangement is fair from a financial point of view, has unanimously approved the arrangement. Directors and executive officers of Neovasc and related parties, holding an aggregate of approximately 9.23% of the Neovasc shares currently outstanding (on a non-diluted basis) have entered into support and voting agreements with Shockwave.
Shockwave Medical Preliminary Fourth Quarter and Full Year 2022 Revenues and Full Year 2023 Revenue Guidance
Preliminary unaudited revenue for the fourth quarter of 2022 is expected to be between $143 million and $144 million, an increase of 70% to 71% compared to the fourth quarter of 2021. Fourth quarter 2022 U.S. Coronary preliminary unaudited revenue is expected to be between $81 million and $82 million
Preliminary unaudited revenue for the full year 2022 is expected to be between $489 million and $490 million, an increase of 106% to 107% compared to the full year 2021. Full year 2022 U.S. Coronary preliminary unaudited revenue is expected to be between $288 million and $289 million
Shockwave Medical projects revenue for the full year 2023 to range from $660 million to $680 million, which represents growth of approximately 35% to 39% over 2022.
Shockwave will provide more detail and discuss full financial results on its fourth quarter 2022 earnings conference call on February 16, 2023.
Conference Call
Shockwave Medical management will host a conference call today at 8:30 a.m. ET to discuss its definitive agreement to acquire Neovasc.
Investors interested in listening to conference call may do so by dialing (877) 423-9813 for domestic callers or (201) 689-8573 for international callers, using conference ID: 13735529. Live and archived webcasts of all earnings events will also be made available at https://ir.shockwavemedical.com.
About Shockwave Medical, Inc.
Shockwave Medical is focused on developing and commercializing products intended to transform the way calcified cardiovascular disease is treated. Shockwave Medical aims to establish a new standard of care for the interventional treatment of atherosclerotic cardiovascular disease through differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which the company refers to as Intravascular Lithotripsy (IVL). IVL is a minimally invasive, easy-to-use and safe way to significantly improve patient outcomes. To view an animation of the IVL procedure and for more information, visit www.shockwavemedical.com.
About Reducer
The Reducer is CE-marked in the European Union for the treatment of refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle, despite treatment with standard revascularization or cardiac drug therapies. It affects millions of patients worldwide, who typically lead severely restricted lives as a result of their disabling symptoms, and its incidence is growing. The Reducer provides relief of angina symptoms by altering blood flow within the myocardium of the heart and increasing the perfusion of oxygenated blood to ischemic areas of the heart muscle. Placement of the Reducer is performed using a minimally invasive transvenous procedure. While the Reducer is not approved for commercial use in the United States, the FDA granted Breakthrough Device designation to the Reducer in October 2018, and it is being studied in the COSIRA-II clinical trial.
About Neovasc Inc.
Neovasc is a specialty medical device company that develops, manufactures, and markets products for the rapidly growing cardiovascular marketplace. Its products include Reducer, for the treatment of refractory angina, which is under clinical investigation in the United States and has been commercially available in Europe since 2015. For more information visit: www.neovasc.com.
Preliminary Financial Results
Shockwave Medical’s audited financial statements for the year ended December 31, 2022 are not yet available. Accordingly, Shockwave Medical’s preliminary financial results are an estimate and subject to the completion of Shockwave Medical’s financial closing and other procedures and finalization of Shockwave Medical’s consolidated financial statements for the year ended December 31, 2022, including the completion of the audit of Shockwave Medical’s financial statements. Accordingly, actual financial results that will be reflected in Shockwave Medical’s Annual Report on Form 10-K for the year ended December 31, 2022, including audited financial statements, when they are completed and publicly disclosed may differ from these preliminary results.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 including, but not limited to, statements related to the expected date of closing of the proposed transaction and the potential benefits thereof; the potential advantages of the Reducer; Shockwave’s preliminary unaudited financial results for the three months and year ended December 31, 2022; and Shockwave’s outlook for the year ending December 31, 2023. All statements, other than statements of historical facts, are statements that could be deemed forward-looking. In some cases, you can identify these statements by forward-looking words such as “may,” “might,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue,” and similar expressions, and the negative of these terms. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current plans, expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware.
Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among others: whether the acquisition of Neovasc is completed; whether the COSIRA-II clinical trial is completed and achieves its endpoints; whether the Reducer receives FDA approval for the treatment of angina; the completion of Shockwave’s 2022 financial statements; Shockwave’s 2023 operational and financial performance; and other risks and uncertainties discussed in our filings with the Securities and Exchange Commission (SEC), including in Part I, Item IA - Risk Factors in our most recent Annual Report on Form 10-K filed with the SEC, and in our other reports filed with the SEC. Except to the extent required by law, we do not undertake to update any of these forward-looking statements after the date hereof to conform these statements to actual results or revised expectations.
Additional Information and Where to Find It
This communication is being made in respect of a proposed arrangement involving Shockwave Medical, Inc. and Neovasc Inc. Further details of this transaction will be included in a management information circular to be mailed to Neovasc shareholders in accordance with applicable securities laws. Copies of the arrangement agreement and the information circular will be filed with Canadian securities regulators and will be accessible on SEDAR at www.sedar.com. The information circular and this communication are not offers to sell Neovasc securities, are not soliciting an offer to buy Neovasc securities in any state where the offer and sale is not permitted and are not a solicitation of any vote or approval.
Media Contact:
Scott Shadiow
+1.317.432.9210
sshadiow@shockwavemedical.com
Investor Contact:
Debbie Kaster
dkaster@shockwavemedical.com
Primary Logo
Would you care to share your thoughts why?
I didn’t think it could be run up with nothing.
Neovasc Reducer Obtains U.S. Outpatient Reimbursement
November 08 2022 - 09:05AM
GlobeNewswire Inc.
Alert
Print
Share On Facebook
via NewMediaWire -- Neovasc Inc. (“Neovasc” or the “Company”) (NASDAQ, TSX: NVCN) today announced that the Centers for Medicare and Medicaid Services (“CMS”) has assigned the Neovasc Reducer™ (“Reducer”) implant procedure a new outpatient reimbursement code payment status indicator, enabling U.S. hospitals to be reimbursed for the device and implant procedure.
Effective January 1, 2023, the implantation of the Reducer in an outpatient setting is assigned to Healthcare Common Procedure Coding System (“HCPCS”) code 0645T and payable under the Ambulatory Payment Classifications (“APC”) Code 5194, Level 4 Endovascular Repair. APCs are the U.S. government’s method of paying for outpatient services for the Medicare and Medicaid programs. The new classification enables the device and procedure to be reimbursed in the current COSIRA-II clinical trial single arm registry and upon potential commercial approval in the United States. The randomized arm of the COSIRA-II clinical trial previously received reimbursement approval in the United States under a dedicated HCPCS code (C9783).
“Today’s news is another important step in securing coding, coverage, and payment for the Reducer in the United States. We now have adequate reimbursement for the Reducer, in the CMS population, for both inpatient and outpatient procedures, both during the COSIRA-II Clinical Trial, and upon potential commercialization in the United States,” stated Neovasc President and Chief Executive Officer Fred Colen. “Our reimbursement journey has been remarkably successful around the world. We are beginning to see broader adoption in markets where we have successfully obtained reimbursement and look forward to continued rapid growth and commercial expansion.”
Neovasc began a comprehensive reimbursement program in 2019 to establish all the necessary components for diagnosis and treatment of refractory angina in the United States. The Company has worked tirelessly with its physician advisors, consultants, CMS, the American Medical Association, and multiple cardiology societies to secure all the necessary approvals and codes for the diagnosis of refractory angina and the implantation of the Reducer in both inpatient and outpatient settings.
Neovasc Announces Third Quarter Financial Results and Provides Corporate Update
4:06 pm ET November 10, 2022 (Newsfile) Print
Vancouver, British Columbia and Minneapolis, Minnesota--(Newsfile Corp. - November 10, 2022) - Neovasc Inc. (NASDAQ: NVCN) (TSX: NVCN) ("Neovasc" or the "Company") today reported financial results for the third quarter ended September 30, 2022.
Recent Highlights
Generated revenues of $923,000, a quarterly record and a year-over-year increase of 31% compared to the third quarter of 2021.
Accelerating acceptance of Reducer in the UK and French markets, with France the largest volume market in the quarter.
Continued enrollment in the COSIRA II pivotal trial, with enrollment expected to be complete in the first half of 2024 and initial data readout in the second half of 2024.
In July 2022, the Company received approval to expand the scope of the COSIRA II trial with a registry to include patients suffering from angina with non-obstructed coronary artery disease, so-called ANOCA patients.
Released preliminary data at the Transcatheter Cardiovascular Therapeutics conference in September, showing the Reducer's benefits to ANOCA patients.
Received US outpatient reimbursement by CMS for the Reducer Therapy, effective January 1, 2023.
"I am pleased to report on the tremendous progress the Neovasc team has made in the third quarter towards advancing our value creation strategies, despite a COVID surge that impacted procedure volumes and currency exchange fluctuations that impacted revenues in the quarter," said Fred Colen, President and Chief Executive Officer. "Despite these headwinds, Neovasc nevertheless achieved another quarter of record revenues as we continue to benefit from the successes in getting the Reducer reimbursed in the UK, France, and in the US as part of the COSIRA II trial. We now have adequate coding, coverage and payment to support full reimbursement for the Reducer, in the CMS population, for both inpatient and outpatient procedures (effective January 1, 2023), both during the COSIRA-II Clinical Trial, and upon potential commercialization in the United States. Additionally, we have developed the new ICD-10 diagnosis code that became effective October 1, that established "refractory angina" as a condition distinct from other, less severe, forms of angina. Furthermore, we continue to provide leading clinical data, this time demonstrating benefits of the Reducer therapy in yet another refractory angina patient population, currently without good treatment options; in September 2022, we announced preliminary data supporting the benefits of the Reducer therapy to patients with angina and non-obstructive coronary artery disease, so-called ANOCA patients. I look forward to sharing further exciting updates in future quarters."
Financial Results for the Third Quarter Ended September 30, 2022
Revenues increased by 31% to $923,000 for the three months ended September 30, 2022, compared to revenues of $703,000 for the same period in 2021. The overall gross margin for the three months ended September 30, 2022 was 76%, compared to 77% gross margin for the same period in 2021.
Total expenses for the three months ended September 30, 2022 were approximately $7.4 million compared to approximately $7.3 million for the third quarter of 2021, representing an increase of approximately $181,000 or 2%, substantially due to an increase in employee expenses as we accrued for a portion of annual bonuses that were previously not accrued, but were incurred, in the prior period and an increase in other operating expenses related to the COSIRA-II study, offset by a decrease in non-cash share-based payments, a decrease in director and officer insurance expenses and a decrease in litigation expenses.
Operating losses and comprehensive losses for the three months ended September 30, 2022 were $6.7 million and $8.2 million respectively, or $3.00 basic and diluted loss per share, as compared with $6.7 million operating losses and $6.9 million comprehensive loss, or $2.79 basic and diluted loss per share, for the same period in 2021. The increase of $16,000 in operating losses can be explained by a $181,000 increase in operating expenses offset by a $165,000 increase in gross profit.
As of September 30, 2022, the company had $31.3 million in cash and cash equivalents.
As of November 8, 2022, subsequent to the effect of the share consolidations, the Company had 2,746,625 common
I still expect this stock to go to 40 or 60 in a day or they get bought out for 80 dollars a share or more that’s why they are not worried about stock price they have enough money til trial over and money they are making in Europe
They made them redo a safety study in USA. Did not like the ones done in Europe. That’s why once approved will cost a lot more for product. In Europe successful and improving quality of life. They should have approved drug but probably not til after study I think march next year. You will see revenue from product in USA because we have approved procedure codes for reimbursement already but being used compassionate and in studying we are getting paid. I still have mine but at one time over a million before split
zeusgodmd, Are you still following NVCN? It sure has been dead. Any idea on the FDA approval?
Company has 47 million in cash 2.8 million shares at 5 dollars so 15million dollars in common float. How does this price make sense
Company’s going to to be sold to highest bidder. Let’s go Boston
Maybe fund managers can buy now above 5 dollars. Maybe Boston scientific will take a chunk. Somebody has given them the iggy to do it this way I’m sure.
Only 2 million shares on the common is crazy company going to get bought out
When they do a reverse split someone will buy them out at around 10 million shares on the float
OK now I know. Thank you. As soon as the FDA, approves it'll take a jump and you'll then see BSX, Edmonds and others start a bidding war. That's what I'm waiting for. To the moon Alice!
When you have cms center for Medicare services number that is a code that Medicare will reimburse the cause for the procedure like putting in a stent or pacemaker has a cost of reimbursement so even if not approved Medicare will still pay if certain criteria per the procedure done so once approved off to the races. Sit come gold mine coming probably a 20 or 30 bagger once approved will be 20 or 30 dollars if you sit tight but trade as you may
I'm sorry zeusgodmd, what is a cms reimbursement code?
Since when does the FDA, have common sense? Safety symptom relief from other countries, why should they listen to inferiors? After all
they are the FDA
Good Luck 2 You!
They have a cms reimbursement code we are good only need approval maybe the will see the safety and symptom relief out side the USA and how much money in hospitalizations they would save but oh well
It's all up to the idiots at the FDA shitting on there thumbs.
They like to rotate.
Crazy stock needs reducer approval then off to the races. Should continue mitral valve and partner with Boston scientific not sure why they have drowned the stock.
Matter of time USA catches up to Germany pretty sad health care better in Europe
Looking good today. Time to double down
Thank you! Been with $NVCN for a long, long time - Hopefully, we are close! Good luck to you as well and thank you, once gain, for posting and responding!
rrao11, just above the postings yesterday was Cosira-11 trial in refractory angina. Today news is another conference with Fred Colen. Hopefully this applies more pressure on the lazy FDA. Good luck to you.
Sorry - I thought there was more good news this morning! Thanks
What good news? Please elaborate! TIA
At 4:20 this morning GOOD NEWS came in. After 3 hours no one posted, I'd thought it should be mentioned.
FDA, needs to stop doing the white house bidding on this virus and pass NVCN. At least some people who have heart problems will live longer with less pain.
This is another reason I never vote democrat!
They invested 60 million in this company 5 years ago buy all of the shares for 40 million
Stays down here will get bought out cheap. Hopefully Boston scientific is watching.
P U a bunch of NVCN. Placed another order @ lower ptice.
If it where Boston scientific or Edward would have went through after a payoff. Canadians don’t know how crooked USA is. Don’t worry we will be owned by one of them if price per share goes down any further it’s too cheap not to get bought out
Remember how the FDA told Fred too make certain changes, he did. FDA likes too play games with people lives. These are not chemicals, people are being buried.
I like your response time. However, your wrong, it's not the tax season reason. It's because the FDA is placing there ammo on Fauci and the WH. This should have BSX or Edwards written all over it. If NVCN should drop in the next couple of days, I'll be all over it.
Think stock should be at a minimum 4$ a share is USA gives reducer a ok you will see that otherwise slower growth just in European markets but alot more smokers in Europe so elevated heart disease and angina. I still hold 800000 shares if that tells you where I think we are going. I would pick up alot it’s down because of end of the year tax selling.
zeusgodmd, your last post was on 10/22/21. Wondering if you've dumped or still holding. What are your thoughts on NVCN. Thinking of getting back in. Thank you.
Looking strong today something must be getting ready to get approved
I don’t know what from maybe one of these guys that paid 2$ a share going to recoup at these low prices
This company can not catch a break. EW will buy them out on the cheap and have both mitral devices. Maybe Boston scientific would set up before this. The reducer gets some us in United States and someone will take the company for there devices
Let’s see if they stay on there script today or give up some good info on approval updates and progress on mitral valve in USA should be granted for fast track approval process by design
|
Followers
|
185
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
11968
|
|
Created
|
12/22/13
|
Type
|
Free
|
| Moderators | |||

March 29th, 2018 3:07 AM EDT
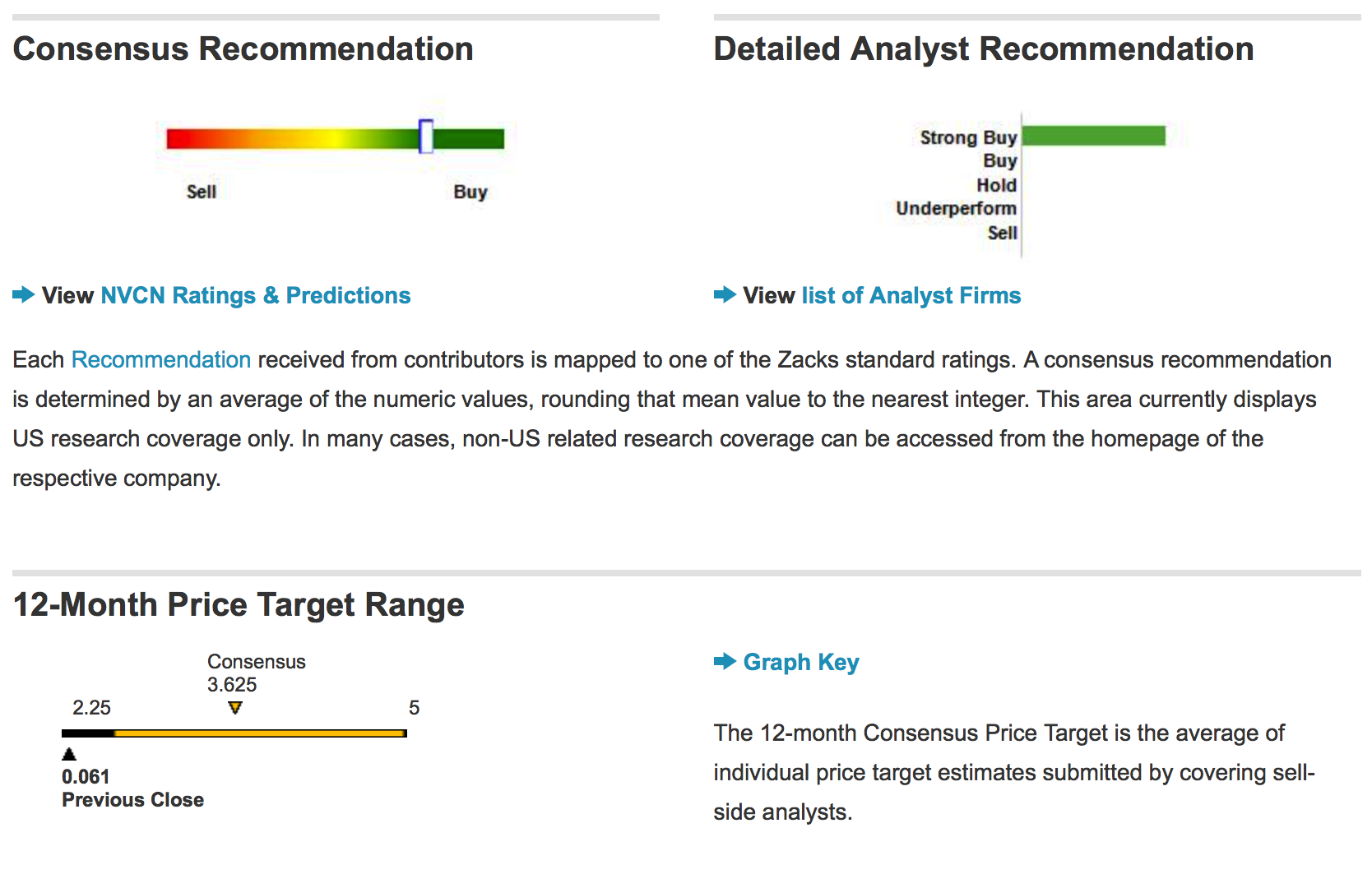
Neovasc Inc. PT updated to $2.00 at Leerink Partners, states that 'Tiara Clinical Trials Continue to Progress' Maintains 'Outperform' Rating.
https://www.streetinsider.com/Analyst+PT+Change/Neovasc+%28NVCN%29+PT+Lowered+to+%242+at+Leerink+Partners%3B+Tiara+Clinical+Trials+Continue+to+Progress/13997143.html
March 29th, 2018
Canaccord Genuity Updates Neovasc Inc (US) (NVCN) Price Target to $0.50 and has a "Buy" rating.
https://registrarjournal.com/2018/03/29/canaccord-genuity-lowers-neovasc-inc-us-nvcn-price-target-to-0-50.html




Neovasc Inc. (Nasdaq: NVCN), a front-runner in minimally invasive transcatheter mitral valve technologies today announced that its board of directors (the "Board") has appointed Fred A. Colen as president and chief executive officer effective immediately. This leadership transition is a key element of the Company's strategy to support plans for the commercialization of its new-to-market Reducer™ ("Reducer") product in Europe and the advancement of its Tiara™ ("Tiara") mitral valve clinical program. Alexei Marko, Neovasc's outgoing CEO, will maintain his role as a Director on the Neovasc Board of Directors and continue to serve as an advisor to the Company.
"After ten years building Neovasc, I am convinced that now is the time for new leadership to take the Company to the next level. I am especially pleased that an industry leader of Fred Colen's caliber has accepted this opportunity," said Alexei Marko, outgoing CEO of Neovasc. "I look forward to my continued involvement as a board member."
Fred Colen has contributed to many significant turnarounds in his career, including the post-acquisition Guidant Company, which became the CRM division of Boston Scientific, a firm with which he held progressively senior executive roles over 11 years, including Chief Technology Officer from 2001-2008 and Member of the Executive Committee from 2001-2010. During his tenure at Boston Scientific, Mr. Colen is credited with numerous successes. As President of the company's Cardiac Rhythm Management (CRM) Group his team regained trust and confidence in the division's implantable pacemakers, leads, defibrillators and re-synchronization devices, increasing annual product revenue growth by over 10% in a flat US market and growing global divisional operating income from below 10% to 25% of sales, exceeding the planned annual free cash flow goals. As Chief Technology Officer, he led the development and global commercial launch for the Company's first- and second-generation implantable drug-eluting coronary stents (the Taxus Express and Taxus Liberte), leading to global market leadership with incremental revenues of US$2 billion annually. The Taxus Express market introduction is viewed as one of the most successful launches ever in the medical device industry.
Also note:
$BSX Boston Scientific laid off 300 employees just recently on March 27th, 2018 , perhaps to make room for the takeover of $NVCN ?? Who knows?? They already own 15% of NVCN.
source: https://www.massdevice.com/boston-scientific-lays-off-nearly-300-from-valencia-neuromod-facilities/

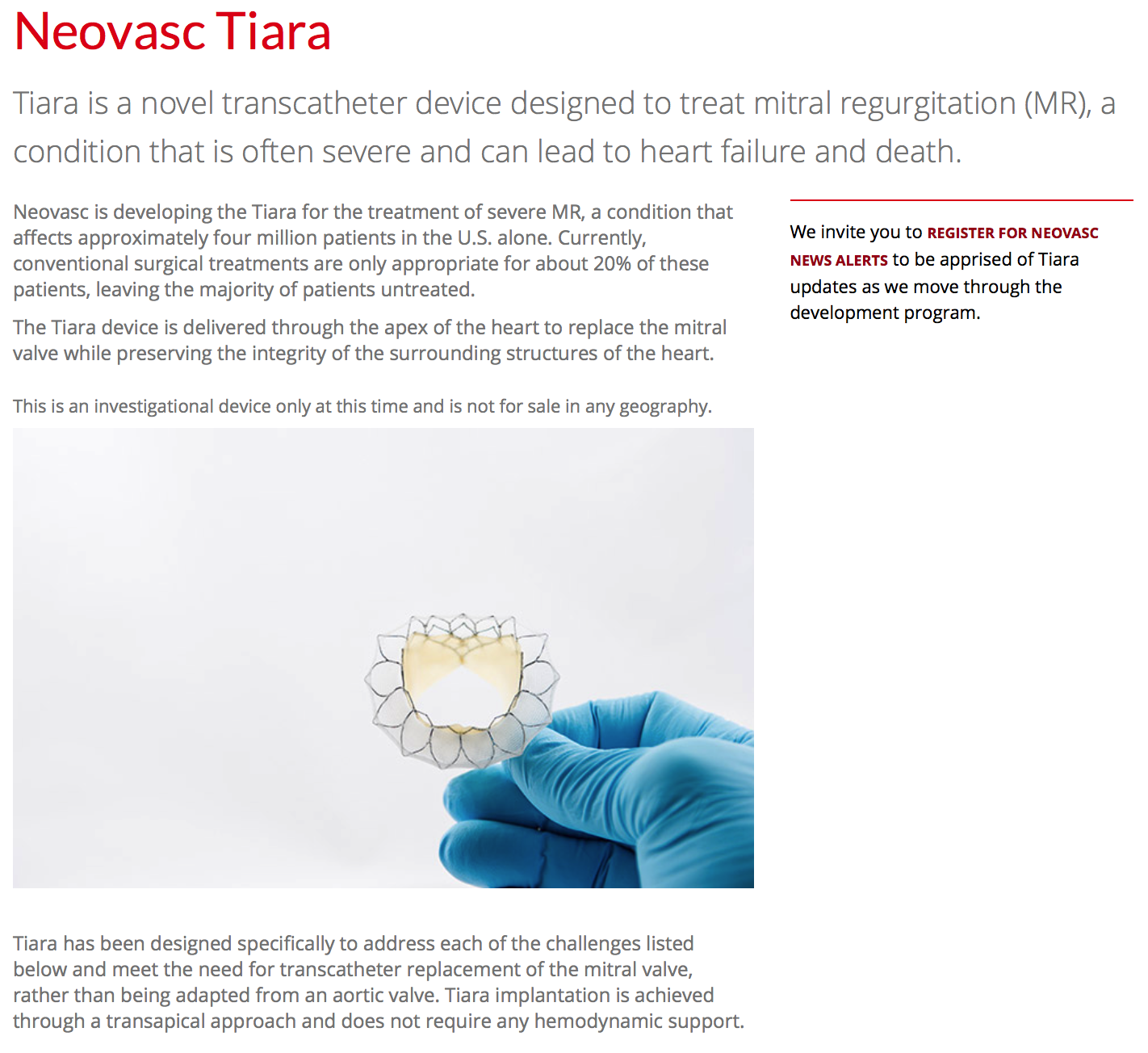
Our products include the Tiara™ technology in development for the transcatheter
treatment of mitral valve disease, the Neovasc Reducer™ for the treatment of
refractory angina.
A Canadian Biotech company, headquartered in Vancouver, B.C. Canada, we are a
publicly traded company, listed on NASDAQ: (NVCN) and the Toronto Stock
Exchange TSX: (NVC)


| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
