Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
I don't know.
But I know that the company did not inform me/us sufficiently.
Fire sale for cheap I guess? What a huge disappointment.
They liquidate:
https://shab.ch/#!/search/publications/detail/ea07e4c0-e37b-4217-b4a0-ad4445486451
Kempers is liquidator.
I would expect him to inform us if there is something to liquidate. And: What's about NIH?
Should turn Alt Reporting any day now.
Thanks siar. It says, "Mymetics has provided its commitments
to this project by delivering the vaccines, which are tested in non-human primates."
The contract ends April 30, 2024. I wonder if the results will be listed on the NIH website or will MYMX release them. I guess it's possible we may not know for some time. Who owns the rights to the vaccine NIH or MYMX. If NIH is happy with the results, what do you think would be the next step. Would it become part of their portfolio? I don't know how MYMX would be able to continue working on a vaccine unless they worked with a larger company in a possible buy out.
Just checked it. It does not say, the results will be released in April, it says testing or the end of the project is April 30, 2024.
You will find that and details about it in the 10-Q from 11/20/2023 at the heading "NIH", e.g. "The Project with ULL under the NIH grant for our HIV vaccine is in its final year that will end on April 30, 2024. Mymetics has provided its commitments to this project by delivering the vaccines, which are tested in non-human primates."
I think, release could be before that date, after or even never, if Mymetics just sells that all.
By the way: Still some people seem to believe that Mymetics is not yet done completely. I always see bids for almost buying all outstanding shares (for nearly nothing).
siar, where did you read the HIV were going to be released in April. I don't recall seeing that. That could possibly provide some good news. Thanks.
I hope that’s the case. It’s frustrating they couldn’t make a deal earlier with so many patents and tech. But we just have to wait it out now.
Thanks for posting that, AhsokaXC. A small group of us public shareholders left … we remain in patient waiting mode. It’s very hard to believe that MYMX is not “in the running” for some kind of merger/buyout. Their portfolio and patents are just way too valuable to throw away. Haven’t seen any selling by insiders, and they know far more than we do.
As Siar notes, the HIV study results next month may prove a catalyst to MYMX news.
Yes, it was another company.
Now I am waiting for the HIV results, in April, if I remember correctly.
Actually a company has to wait 90 days after filing the Form 15-12G before the ticker is deregistered. They can't speed that up.
I don't think the ticker would go to Expert Market anyway until OTCmarkets.com realized they didn't file an annual report. That would be March 31st or right afterwards, coincidentally also 90 days.
I never expected a complete wipeout here. How can all their tech and patents be totally worthless? It seems more like an inside move to feather someone's nest.
I'll be filing a SEC complaint like most here but it's difficult to press the case unless you can point to egregious and blatant fraud. I don't expect much to come of it,
Happy New Year and Best to all
So today is the day MYMX shut down as a public company. How can remaining shareholders communicate among ourselves; is there a chat room available to us?
assume you found it: MYMXD
MYMX one for 2,000 reverse split:
https://otce.finra.org/otce/dailyList?viewType=Symbol%2FName%20Changes
By it's company name or just put it in your favourite boards list.
Then how would one access it?
I guess they are offering to buy stock. So if you can get a 100% return immediately maybe that’s the play? But I think there’s more going on behind the scenes.
I think this message board will remain available, but surely not accessable by means of the ticker symbol.
Now 4.3 million on bid.
Agree with your sentiments. Will this site remain available for us to communicate as MYMX goes private?
New 8-K.
"[...] to effectuate the Reverse Stock Split, and such amendment was effective as of 12:01 a.m. on December 28, 2023."
While trading?
"On or about January 2, 2023, the Company is filing a Form 15 [...]"
I am disappointed with the performance of Mr. Kempers, so now I could be a bit sarcastic and praise that he is once ahead of all others.
And still I would like to know who is willing to buy 3.7 million shares a few days before going private.
Merry Christmas everyone. I hope you all have a wonderful day and also hope we get good news soon.
I think the creditors have first right of access to the assets.
And it seems to be clear, that negotiations are going on, or are just finished. For cost reduction the company has to go private today or next week. This is already a tight timeframe.
I am curious about the outcome of Mr. Kempers "gamble". He seems to have updated, better to say, cleared and hided a bit of his linkedin account.
In a few days we will see whether we could roughly get back our investment or loose everything - I think there's almost no other option.
MYMX’s two major creditors - Round Enterprises LTD and Eardley Holding, AG - have clauses for over a decade stating that their loans are “secured against all property of the Company”. Does that relate here?
Not sure siar. It says the assets are held by a secured creditor. I haven't seen any filing saying MYMX assets are being held by a creditor.
It seems the last 5 filings are just withdrawing stock options from years ago. I read a notice of effectiveness was when they want to sell securities, but I guess it's to sell or withdraw. Is that correct?
Just read this and wondering if there could be a relation to MYMX
https://www.otcmarkets.com/stock/BVAXF/news/BioVaxys-in-Non-Binding-Discussions-for-Major-Immunotherapeutics-Technology-Acquisition?id=424591
Never under estimate a shareholder
Thanks, that’s good to hear. It seems both MYMX insiders and us shareholders are in flux…it will be very helpful to stay in touch as events unfold.
I've already filed a complaint with the SEC. Good advice for everyone here to do so.
Interested Parties Should File @ SEC Website. More people who file will bring attention to MYMX.
This MB will never go away. It's not driven by the status of the MYMX ticker.
witness KKUR, SPNG, et al -- tickers that are long gone but the iHub MB lives on.
extrinsic55, if you're starting a class action that's great. I would certainly join in. I have called a couple lawyers but haven't found one to take the case yet. It doesn't seem like enough interest to start another way of communicating. If something important comes up after the, going private. I'll post on CTLT.
Here's a question maybe one of you can answer. If MYMX as an OTC stock decided to go bankrupt, would they still have to close down subsidiaries, file end of year and quarterly reports, and why go through a reverse stock split if they were going to fold up the company anyway. I've never felt the insiders have been honest with the shareholders this whole time. Once they go private, even if they make a deal, I don't think they'll treat us fairly.
That’s correct. We’re waiting on that outcome. It states they can decide to change their mind.
Would join a class action. Interesting that the SEC quarterly report notes that the reverse split may or may not occur as presented. What is behind the scene seems to be playing out at present.
Templar / All good luck. The process takes time. Lawyer will contact all shareholders in due process. I can tell you this. Going after the money. Not the company but the players. Especially Kemper and Stahlein. Waiting on Mymetics next step before action. This will take years if successful is helpful to stockholders to a point. The leads or person or persons who file come out whole or ahead. The other stockholders get a piece. Its best to be the leads in filing so if your contemplating action find a good attorney. I can only tell you my experience. 4 years but happy with conclusion on my lawsuit 10 years ago.
Hmmm…interesting!
Doesn’t seem like the board is going away and shares are still trading it seems.
I suspect some people bet on a takeover tomorrow. That would explain the trading volume in recent weeks.
I know that some posters on other stocks have chosen to form private chat spaces from time to time. Does anyone here know how to set that up, either on stocktwits or another chat board, and how to join it? I’m okay with ctlt as a stopgap, but will need something more focused on our status and concerns in the near term.
I've called a couple lawyers but haven't heard back yet. extrinsic55, do you have a lawyer and are you planning a class action lawsuit?
castaways, I would like to find a way to communicate to other shareholders as well. If anyone has ideas on how to do so, please let us know soon as our time here is running out. If they go private on the 11/16 will this board shut down right away?
How will we communicate after 11/16? I would like to stay informed of shareholder activity regarding MYMX.
DEF 14C out. Looks like Nov. 16th will be the day of infamy.
In reading the OTCQB requirements again and then reading the 8K, it was the BID price that closed below .001, not the stock price.
OTCQB requirements are all about the closing bid price, not the last price paid (closing price).
Count me in also.
|
Followers
|
86
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
27643
|
|
Created
|
09/25/02
|
Type
|
Free
|
| Moderators | |||

http://www.mymetics.com/
COMPANY OVERVIEW
https://www.mymetics.com/files/8116/2081/0445/May21-Mymetics_Overview.pdf
Mymetics Corporation is US registered biotechnology company with its main offices in Switzerland and the Netherlands.
Focused on developing next generation preventative vaccines for infectious diseases.
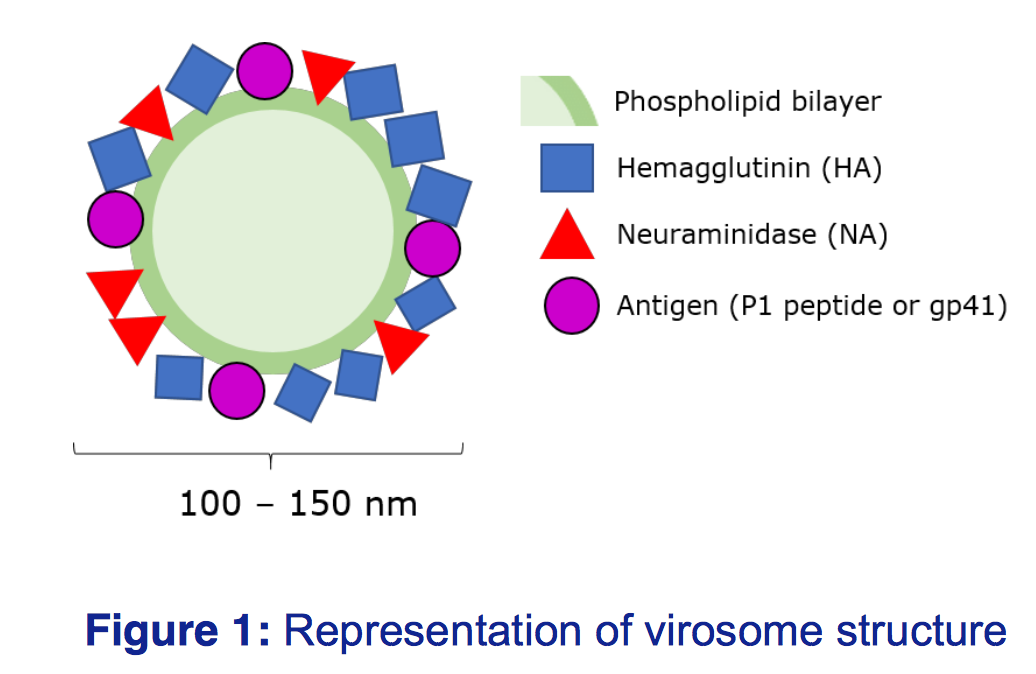
Mymetics core technology and expertise are in the use of virosomes,
lipid-based carriers containing functional fusion viral proteins in combination with rationally designed antigens and membrane proteins.
Objective: "Build small / medium size innovative R&D virosome vaccine company with strong
partnerships, Phase II – III clinical vaccine pipeline and have optionality for M&A or sale."
Current Share Structure:
Outstanding Shares: 303.7 million (as of 01/18/2019 unchanged since 2014)
Floating Shares: 79.8 million* (a/o 01/18/19)
(https://www.otcmarkets.com/stock/MYMX/profile)
Rationale and Impact of MACIVIVA
With few exceptions, commercialized vaccines are generally delivered by injection through the intramuscular or subcutaneous route.
Vaccines contain immunogens classically found within a large variety of biological compounds such as peptides, proteins, glycoproteins and sometimes carbohydrates and lipids.
These immunogens may trigger the immune system for producing antibodies and/or cytotoxic T cells for preventing the pathogen transmission or blocking and/or slowing down the disease progression.
However, these vaccines generally exist as liquid formulation that are inherently prone to physical and/or chemical modifications. The cold chain storage is still fundamental for preserving the
bioactivity of most liquid and freeze-dried vaccines. For reconstituted freeze dried vaccines, they harbor important instability and must be used within hours and kept refrigerated. Vaccine degradation generally takes place
during shipment and/or storage of liquid or lyophilized products, which may affect the immunological properties of the immunogens, with unwanted immune responses or insufficient immune protection.
There is growing evidence that solid dosage formats (e.g. powder form) for vaccines may offer several advantages over the liquid formulations, such as the prevention of molecular motion and shear-induced degradation,
and slowing down modifications and degradation reactions involving water and oxygen radicals, resulting in improved stability, enhanced shelf-life of vaccines and greatly simplified logistics.
MYMETICS BV
Expertise: R&D on virosome formulations
Project responsability: Investigating and compiling the results about the physical and biochemical properties of the virosome-based vaccines obtained by spray-drying and lyophilization.
MYMETICS SA
Expertise : Non-GMP and GMP virosome production, clinical development
Project responsability: Excipient selection for liquid virosomes, supervising the non-GMP and GMP manufacturing of the liquid virosomes and development of analytical methods.
UPPERTON LIMITED
Expertise: Non-GMP and GMP Spray drying
Project responsability: Identification of excipients and experimental conditions suitable for virosome spray drying, production of non-GMP and GMP powder forms for nasal and oral delivery.
CATALENT U.K. SWINDON ZYDIS LIMITED
Expertise: Zydis technology for fast-dissolving tablet, world leader in drug formulation and distribution
Project responsability: Identification of excipients and experimental conditions suitable for virosome lyophylization, according to the Zydis technology, non-GMP and GMP tablets for sublingual delivery.
CHIMERA BIOTEC GMBH
Expertise: Ultra sensitive immunoassays development and bioanalysis based on Imperacer® (Immuno-PCR) technology.
Project responsability: Immunogenicity study in animals with spray-dried and lyophilized virosomes. Imperacer® immunoassay development and evaluation of the vaccine-induced antibody response.
BACHEM AG
Expertise: R&D, non-GMP and GMP manufacturing of API, world supplier
Project responsability: Process Development and manufacture of peptide P1, GMP-grade, including development and validation of analytical methods.

Vaccines are poorly accessible in developing countries
Vaccines require cold-chain storage and are often delivered by injection, which is undesirable, less safe and more expensive to administer.
Developing thermostable solid form vaccines through non-invasive routes may represent a long-term global solution to the vaccination challenge (Amorij, 2008).
Virosomes are an efficient vaccine delivery system
Virosomes are spherical, unilamellar lipid-based carriers, intercalated with functional glycoproteins to reflect the natural virus, however the lack of viral RNA means there is no risk of infection
(Figure 1). Virosomes can be tagged with different antigens and adjuvants, meaning they can be tailored to target different viruses, and offer increased immunogenicity over inactivated viruses.
Currently, virosomal influenza vaccines are only available in liquid form (Amorij, 2008).
Spray drying can produce dry powders for a range of dosage forms, including inhaled or nasal drug delivery.
A dry powder is formed when a liquid feed solution or suspension is atomised using a spray nozzle, and rapidly dried using hot air. However, while the drying process is gentle due to evaporative cooling,
there is still the potential to stress and inactivate vaccine components. It has been found that subunit and live-attenuated vaccines (and other delicate molecules such as proteins)
can be protected during processing b by incorporating them in an amorphous sugar matrix, which also offers longer term stability during storage (Kanojia, 2016).
A method has been developed to produce a powder form of virosome based influenza vaccine using spray-drying.
Formulations have been optimised for oral and nasal delivery.



Virosomal technology is approved by the FDA for use in humans, and has a high safety profile
Virosomes are biodegradable, biocompatible, and non-toxic12
No disease-transmission risk
No autoimmunogenity or anaphylaxis10
Broadly applicable with almost all important drugs (anticancer drugs, proteins, peptides, nucleic acids, antibiotics, fungicides)
Enables drug delivery into the cytoplasm of target cell
Promotes fusion activity in the endolysosomal pathway
Protects drugs against degradation
Intellectual Property
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
