Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
lol...probably on of the pumpers here with a lot of cheap shares
Seems the holders will want a pay to worth their patience, interesting future here for LLBO
That would bring us to a market cap of 4.5 billions. Which requires a well as advanced/ high revenues making company.........
I was shooting for .25. After reading all this I changed my sale orders to 1.25. Thanks Arnie!! Bullish !!
V: We will succeed!! Don't be doubtful! Once it will starts Trading it will rocket up fast!!
I hope there’s a golden rainbow coming for this company even if hope is not a good investment strategy it can be sometimes a generator of the biggest gainers
Cheers!
Vinsterr: I do not. Know how many shares you own; but if you own at least a Million shares, in two more years you'll will probably a multi millionaire !! You have to think Big and Positive!! Remember one cent doubled everyday will amount to over 5 M in 30 days!! Believe and see it!!
Thanks man I appreciate your resume, I’ve been here ever 2 years, it seems I will finally make a couple of bucks out of this..
Hoeever, it could happened sooner!!
The latest update is that we are waiting for the completion of the Audit which could be completed in 1-3 wks! Once the Audit is completed, then it will be followed by the filing of form 10 to be submitted to the OTC and SEC!! Once it is approved by SEC ! I think we will be pink current! Then we are ready for Trading! Probably another additional 3wks!! You have to be patient in this world to succeed!!
Just look at it again to confirm that you have earned it....
lol ok thanks man
Pls read the postings below for the last 5 days!
What are the latest updates on LLBO why the sudden enthusiasm?
I strongly agree!!
a new chapter could be starting ..the past is gone.....positivity is the key in Life
Get series: I have been reading the opinions in this chat for 20 years, I just have never given an opinion why I didn't have any information to contribute. Yes I have lived all these stories these years
Hopefully Acropolis saw how fast PPS Exploded today with LLBO, and once it is PinkCurrent, there are thousands of investors on twitter that will raise the Price quickly. Hopefully they speed up the process(Audit,Form 10, PinkCurrent) if they saw that.
Then you remember the promises Jim Holmes was making 20 years ago, and 15 years ago, and 10 years ago, and even 5 years ago.
Best of luck!
Hope so! Best of luck!
I have been in llbo for 20 years.
I hope to sell them soon.
Greetings from Spain
Luckyyu: Me too! Praying for the success of LLBO and Shareholders is part of my daily prayers!! Do not let anybody steal your dreams and goals!! Believe and see it!!
..2021-07-14..stock price.close@0.0098 volume 159126100 high 0.0117 low 0.0066 ..if something good happends depends how good just a example what to expect plus much more ! numbers are a historical fact and the rest is my opinion
GetSeriousOK - have you not seen all the documents posted on “X” by StockRunner? Things are on the way… soon we will see the outcome! :)
Arnie1 - still praying everyday for the best outcome for us! :)
LOL you guys, somebody bought 200,000 shares at .0008 yesterday... spending $160
and today it was 40,000 at .003.... spending $120
So I wouldn't order that Ferrari quite yet.
You still need Currier to sell the shell, and for that matter the Corporation is still in Default in Nevada.
Look at every single one of Currier's other Custodianship plays. They all suck. Even the supposed success story ILUS went into the toilet. So before you start talking about a penny or a dime or a dollar.... watch the otcmarkets.com page for filings. That will be your first warning that something is actually happening.
This will go to Nasdaq from OTC!!
Luckyyu: when this go to a dollar, it will rocket up quickly!! Especially when there is a merger coming up, hopefully, Sensoria!!
like on a higher volume lol not like now
Ok..lets see what price they are willing to pay...
Ok I didn’t know why I was 6k$ richer today I see now…
Nope, can’t buy!
i think you could buy some now around ..0.0023
well..it all depends of the price...@ 1 cent I think I would sell you like half a mi
If I could find that I would buy it myself to add more to my pot! Just saying! :)
just in case i need like around 1 m more...for trading back& forth
I doubt it… everyone is holding because we all know what’s going on!
i see this ! to 5 cents range..jmo,,,but the mm will try to steal your shares cheap..be carefull
I could buy..did buy some before...any for sale?
Can’t buy in USA yet… only sell.
No way Arnie1…. :)
Luckyyu: do not sell for at least a dollar!
well. lets see..lol
Same should be a good runner
Under .01??? Don’t sell for under .25!!
Looking good! Woohoo!!! Can’t wait for it to start trading in the US!!!
Welcome to Lifeline Biotechnologies Inc. (LLBO) Board!!!
Clinical Trial
In Progress - To Be Completed
** Funding for Clinical Trial
** Vendor delivery of testing materials
Completed for Clinical Trial
** Protocol for testing accepted
** Internal Review Board (IRB) approved
** Contract with hospital executed
Prospective Partner
** Stanford University's Canary Research
Research in blood genomics

The Mastascope is an ultraslim, hand held endoscope designed to enter the breast nipple via one of its milk ducts, thus permitting the physician to visualize the interior aspects of the breast. Because many cancers actually begin within an individual milk duct, these intraductal tumors are visualized easily and can be biopsied. This product has been used in leading breast centers in the United Kingdom and other countries with great success. Lifeline has obtained a CE mark for marketing and distribution in Europe.
Lifeline has developed and tested an ultraslim endoscope to directly visualize the ovarian surface in the search for cancer. This is of great advantage to physicians, because neither MRI, X-Ray nor Ultrasonography can provide accurate assessments of subtle surface changes. Initial reports of clinical usage have been encouraging.
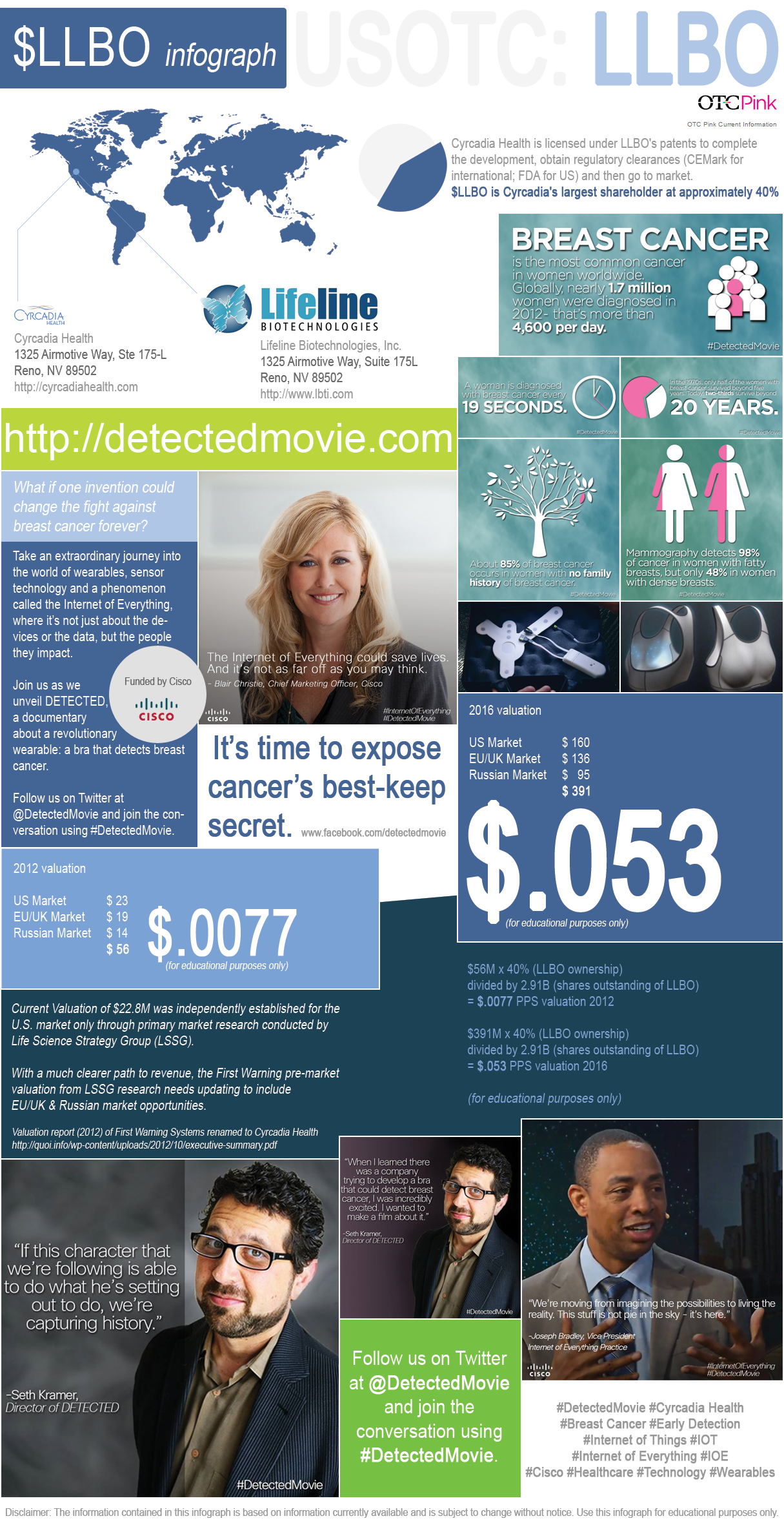
About $LLBO - Lifeline Biotechnologies, Inc.
About Lifeline Biotechnologies:
Lifeline Biotechnologies, Inc. (OTC Market: LLBO) founded in 1994 is based in Reno, NV. LLBO has invested over $10M in a device and process for early detection of breast tissue abnormalities some of which are cancerous or pre-cancer. Three “Proof of Concept” clinical trials of over 500 women have been completed and three patents (one hardware, two software) have been awarded.
Safe Harbor:
This publication includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 27E of the Securities Act of 1934. Statements contained in this release that are not historical facts may be deemed to be forward-looking statements. Investors are cautioned that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from that projected or suggested herein due to certain risks and uncertainties including, without limitation, the ability to obtain financing, successful development of the Company's product or market acceptance of the product and regulatory and shareholder approval for anticipated actions.
http://www.firstwarningsystems.com/ became Cyrcadia Health http://cyrcadiahealth.com/
http://www.lbti.com
$LLBO is Cyrcadia's largest shareholder at approximately 40%.
Transfer Agent
OTC Corporate Transfer Service Co.
52 Maple Run Drive
Jericho, NY 11753
| Shares Outstanding | 3,026,680,967 | a/o Sep 30, 2014 |
| Float | 650,000,000 | a/o Jun 30, 2009 |
| Authorized Shares | 4,950,000,000 | a/o Sep 30, 2014 |
| Par Value | 0.001 |
About Lifeline Biotechnologies:
Lifeline Biotechnologies, Inc. (OTC Market: LLBO) founded in 1994 is based in Reno, NV. LLBO has invested over $10M in a device and process for early detection of breast tissue abnormalities some of which are cancerous or pre-cancer. Three “Proof of Concept” clinical trials of over 500 women have been completed and three patents (one hardware, two software) have been awarded. http://www.lbti.com
Cyrcadia Health founded in 2008, is based in Reno, Nev. The Cyrcadia Health product line is a device and software service that detects breast tissue abnormalities leading to health risk assessment and management including early breast cancer identification. Three clinical trials with over 650 participants have achieved proof of concept and superior outcomes when compared to other diagnostic protocols. Cyrcadia Health technology is exclusively licensed for development, manufacturing and marketing worldwide from Lifeline Biotechnologies, Inc (OTC Market: LLBO). Cyrcadia Health is planning a final, limited clinical trial and a 510k device classification to validate the fourth generation of the FWS product. Cyrcadia Health is preparing to apply for a Euro CE Mark to market in the European Union and Asia Pacific markets. See Cyrcadia Health video, “Breast Cancer Tumor Progression” at http://www.cyrcadiahealth.com/
iTBra in the News
ABC 7 News - February 5, 2015
They also interviewed Rob Royea (Cyrcadia's President) for a feature story on Good Morning America expected to air in the next coming months.

https://mobile.twitter.com/RobRoyea/status/564872820635627520
Could a Bra Actually Detect Breast Cancer?
By Smithsonian Magzine

http://www.smithsonianmag.com/innovation/could-a-bra-actually-detect-breast-cancer-180954612/?utm_source=twitter.com&no-ist

...Cyrcadia prepares to enter the final clinical trials that will be conducted at El Camino Hospital (ECH) in Mountain View, CA and the Ohio State University (OSU) Oncology Hospital in Columbus, OH. The trials have been approved through Investigational Review Boards (IRB's) of ECH and are currently undergoing the review process at OSU. The ECH trial is slated for start before the end of April 2015. The trials are expected to be completed in four to 6 months. On completion Cyrcadia will obtain CE Marking allowing for sales and marketing outside of the USA. On completion of the trials Cyrcadia will be submitting a 510(k) to the FDA. Post clearance, marketing will begin in the USA. International sales are expected in Q4 of 2015 and US sales in Q1 or Q2 of 2016.
It is interesting to note that Dr. Farrar at OSU will be the primary investigator for the trials conducted at OSU. Dr. Farrar headed the original "proof of concept" study that was conducted at OSU.
Stanford University Canary Center for Early Cancer Detection has pledged $187,000 to help fund the equipment for the trials at El Camino Hospital.
They also interviewed Rob Royea (Cyrcadia's President) for a feature story on Good Morning America expected to air in the next coming months.
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
