Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Oops its a vice of mine not to give context...
So what I understood is special about Ionis compounds is that we can treat the bad LDL cholesterol and triglycerides for heart health but this LPA is genetically fixed and cannot reliably be lowered right now.
So this company can do shady business all they want if the results are right and the Novartis partnership hints at that.
I remember some analyst said that the medical societies will quickly get behind screening and treating it if their trials read out positively.
high?
High levels of lipoprotein (a) (Lp(a), pronounced “L-P-little-A”) increase your likelihood of having a heart attack, a stroke, and aortic stenosis, especially if you have familial hypercholesterolemia (FH) or signs of coronary heart disease.May
the Olezarsen/TRYNGOLZA NEJM paper claimed there were ZERO adverse events — perhaps a transient platelet decrease
Later the FDA label reveal 5 such events (12% of patients) in the same study.
All that will be forgotten quickly should the IONS / Novartis ph3 read out positively. Could be the only compound on the market for a while that can significantly lower lipoprotein a.
Lipoprotein a lowering might be one of the big biopharm stories in 2025.
$IONS short squeeze Ionis Pharmaceuticals Inc Nasdaq Ions Short Squeeze
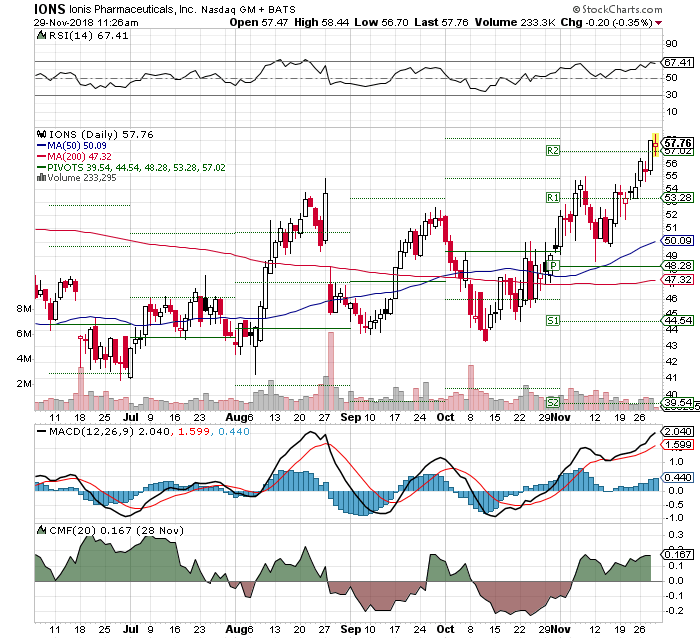
What a beast. Just keeps going up
57 made in the shade. A little pause to regroup and popping 60. Shake, Rattle and Roll just watch the reaction to when this presentation hits the streets:
https://finance.yahoo.com/news/first-class-therapies-using-advanced-120000967.html
Jimmy:
The FDA is seriously underfunded and being pulled in different directions by an ignorant public, ignorant legislators, big money lobbies, and loads of big pharma money.They are struggling.
They do the best they can.
The antisense platform is valid and has some FDA approvals and cured folks as proof.
The FDA is afraid of risk, even in the cases of folks with fatal diseases. The liability attorneys flock like hungry vultures.
The advisory committee recommended approval. A very small number of patents with deadly disease with no other options and the FDA issued a CRL instead of approval. Patients die short sellers get rich. What is wrong with the FDA? Corruption? Or is the Anti-sense platform a fraud? Get a rope. But who should be strung up IONS or the FDA? Right now it is the patients and long stock holders feeling the pain.
Exicure to Present Data at the Cure Spinal Muscular Atrophy Annual Conference in Dallas
Exicure’s three-dimensional spherical nucleic acid containing the nusinersen sequence prolongs survival and reduces toxicity compared to nusinersen in a spinal muscular atrophy mouse model
SKOKIE, Ill. – June 14, 2018 – Exicure, Inc. (OTCQB:XCUR), the pioneer in gene regulatory and immunotherapeutic drugs utilizing three-dimensional, spherical nucleic acid (SNA™) constructs, announced today that Exicure and its collaborators at The Ohio State University Wexner Medical Center will show preclinical data demonstrating the performance of Exicure’s SNA compound designed for use in spinal muscular atrophy (SMA). These data will be presented at the Cure SMA Annual Conference in Dallas, Texas on June 14, 2018.
“Exicure’s spherical nucleic acid version of nusinersen demonstrates increased survival and decreased toxicity in the translationally-relevant SMA mouse model,” said David Giljohann, PhD, CEO of Exicure. “We believe these results are important for developing improved treatments for patients with SMA. These data also suggest that Exicure’s technology platform could potentially create more potent therapies for other disorders of the central nervous system, including Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease.”
At the meeting, Arthur Burghes, PhD, from Ohio State’s Wexner Medical Center, and Exicure will present data from preclinical studies in a SMA mouse model. The poster is titled “Nusinersen in spherical nucleic acid (SNA) format improves efficacy both in vitro in SMA patient fibroblasts and in ?7 SMA mice and reduces toxicity in mice.” The presentation will highlight that Exicure’s proprietary technology:
Prolonged survival by four-fold (maximal survival of 115 days compared to 28 days for nusinersen-treated mice);
Doubled the levels of healthy full-length SMN2 mRNA and protein in SMA patient fibroblasts when compared to nusinersen;
Doubled the quantity of healthy full-length SMN mRNA levels in spinal cord tissue compared to untreated mice;
Mitigated toxicity of nusinersen at the highest dose tested in mice.
In August 2017, Exicure and The Ohio State University established a collaboration to further validate and characterize the pharmacology of Exicure’s nusinersen-SNA compound in mouse models. This collaboration’s ongoing in vivo research is conducted by Dr. Burghes, an internationally known researcher, leading basic and clinical research on SMA and other genetic neuromuscular disorders.
About Exicure, Inc.
Exicure, Inc. is a clinical stage biotechnology company developing a new class of immunomodulatory and gene regulating drugs against validated targets. Exicure's proprietary 3-dimensional, spherical nucleic acid (SNA™) architecture unlocks the potential of therapeutic oligonucleotides in a wide range of cells and tissues. Exicure's lead programs address oncology, inflammatory diseases and genetic disorders. Exicure is based outside of Chicago, IL. For more information, please visit www.exicuretx.com
About Spinal Muscular Atrophy (SMA)
SMA is the most common genetic cause of death for infants. SMA results from the loss of the SMN1 gene and an inability of SMN2 to produce sufficient full-length protein to make up for the loss of SMN1. The SMN1 gene, in a healthy person, produces a full-length protein that is essential to the function of the nerves that control muscles. Without sufficient SMN protein, the nerve cells cannot properly function and eventually die. This leads to debilitating and even fatal muscle weakness.
About Nusinersen
Nusinersen, marketed as Spinraza® by Biogen, is a modified antisense oligonucleotide. In December of 2016, nusinersen was approved by the US FDA for the treatment of SMA in pediatric and adult patients.
Forward Looking Statements
This press release contains forward-looking statements (including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act of 1933, as amended) concerning the Company, the Company's technology, potential therapies, pre-clinical results, and other matters. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. Statements that are not historical facts are forward-looking statements. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: that Exicure's pre-clinical programs do not advance into the clinic or result in approved products on a timely or cost effective basis or at all; regulatory developments; and the ability of Exicure to obtain sufficient funding for its programs and to protect its intellectual property rights. Exicure's pipeline programs are in various stages of pre-clinical and clinical development, and the process by which such pre-clinical or clinical therapeutic candidates could potentially lead to an approved therapeutic is long and subject to significant risks and uncertainties. Risks facing the Company and its programs are set forth in the Company's filings with the SEC. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Media Contact
Karen Sharma
ksharma@macbiocom.com
781-235-3060
So our new drug that gives a small population of doomed children a chance at life, only sold about as many doses as last quarter.
Everyone knew it was a small patient population and another important test of the success of our anti-sense platform.
As a reward, the SP plummets 10%!..A genuinely chicken-ship reaction from the market
Ionis Pharmaceuticals, Inc. (NASDAQ:IONS): Stochastic Analysis
Technical Indicators and Signals
The stochastic oscillator indicator is the important indicator to compare the security price over a certain period of time. The stochastic %K of Ionis Pharmaceuticals, Inc. (NASDAQ:IONS) for the period of 9-Day stands at 91.68% while the stochastic %D for the same period stands at 90.12%.
The 7-Day Average Directional Index ADX (also known as Trend Strength Indicator) of Ionis Pharmaceuticals, Inc. (NASDAQ:IONS) signals “Buy” while other important signals like the 20-Day Moving Average Vs. Price signals “Buy”.
Another important signal of trend and momentum of a security is 20-50 Day MACD Oscillator or Moving Average Convergence Divergence signals “Buy” for Ionis Pharmaceuticals, Inc. (NASDAQ:IONS) while 20-100 Day MACD Oscillator
“Buy”.
http://www.stocksmarketnews.com/ionis-pharmaceuticals-inc-nasdaqions-stochastic-analysis/
So, let's see....
IONS announces a new clinical trial series for a drug that might command enormous , unserved markets;
Collects a fat wad of dollars,
and the PPS sinks over # 3%.
Riiiight! Rational market indeed.
"CARLSBAD, Calif., Oct. 13, 2017 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (NASDAQ: IONS) today announced that it has initiated a Phase 1/2a clinical study of IONIS-MAPTRx in patients with mild Alzheimer's disease (AD). Ionis earned a $10 million milestone payment from Biogen related to the initiation of this study."
Thanks Capecod.
If you want to be on a large board for ionis go to investor village and start posting on the public board there for ionis and then after a while ask if they will let you onto the private board. They let some people on and not others. You would be surprised how much posting they have on the private board and even the public one there has more than here.
That would explain the paucity of postings.
Please let me know if you find one Capecod.
( i'm not far from Big Sur on the other side of the continent.)
Is there a private board on this platform that has more ionis followers? There are a large number of followers and daily postings on a private board at investor village. Would like to join a similar board if there is one on this platform.
And between us we darn near own this board..grin.
I like this company too.
Thanks for the updates Thoro;
Ions has done quite nicely for me over the long run,
and with their promisingly fat pipeline
i'm sure to let it ride.
IONS, AKCA, Akcea and Ionis Announce Submission of Marketing Authorization Application for Volanesorsen to the European Medicines Agency
https://ih.advfn.com/p.php?pid=nmona&article=75321782
Ionis Pharmaceuticals reports phase 1/2 clinical study results with AKCEA-ANGPTL3-L
http://www.reuters.com/article/brief-ionis-pharmaceuticals-reports-phas-idUSFWN1IR0BI
Ionis Pharmaceuticals presents new data at peripheral nerve society meeting to support potential benefit of Inotersen
IONS
http://www.reuters.com/article/brief-ionis-receives-10-mln-milestone-pa-idUSASB0B97F
Ionis receives $10 mln milestone payment from Biogen
http://www.reuters.com/article/brief-ionis-receives-10-mln-milestone-pa-idUSASB0B97F
IONS
Reuters info on Ionis:
http://www.reuters.com/finance/stocks/overview?symbol=IONS.O
Another successful Ph 3 clinical trial result...
"The clinically meaningful benefit in quality of life bring a new energizing level of hope to the amyloidosis community around the world, a community whose critical needs have not been sufficiently addressed," said Isabelle Lousada, president and chief executive officer of the Amyloidosis Research Consortium (ARC)."
And again, the PPS sinks 4%!!!!
WTH ?!?!
I wish we had an effective SEC and less parasites gaming the market!
Not gonna happen in this era of deregulation.
But i'm buying more just to spite them. This is a great company with an enormous pipeline.
7:47 am ET
*Ionis Pharma Reports Earning of $50M Milestone Payment from Biogen Following EC Marketing Authorization for SPINRAZA
Benzinga
7:30 am ET
SPINRAZA(R) (Nusinersen) Approved in the European Union as First Treatment for Spinal Muscular Atrophy
BusinessWire
I don't understand why we are only up 6%?
Yeah, right...You shorts sure can get ugly.
If you did a bit of DD you'd know that in the second half of the trial a new protocol was put in place that prevented the negative outcomes...period.
I bought more.
Now the EU has just granted marketing authorization for this life saving drug for children
Ions down today 9% despite the big PH 3 TTR study meeting primary endpoints.
They had to add monitoring of negative side effects because of 3 deaths part way through. Once that was in place, no more serious problems.
This is the only effective treatment for TTR, an inherited disease that leads to an early death in childhood.
AF tweeted based on the early adverse reactions and drove the price down
I'd guess the FDA pretty much has to approve it.
So now would be an excellent time to pick up more shares...I will.
Chalk up a major point for IONS!
"
Biogen's SPINRAZA(R) (nusinersen) Receives Positive CHMP Opinion for the Treatment of Spinal Muscular Atrophy"
$IONS and $OMER - For growth investors there are two great stocks out there in the biotech sector, according to experts:
Quick funadamental Analysis in this rticle below...
http://mytradingbuddy.com/markets-analysis/my-trading-buddy-markets-analysis-stocks/biotech-stocks-growth-investment/
It's about to get much worse with the new administration and deregulation.
I've been wondering if the prudent thing isn't to get out of the market entirely?
We are a smallish emerging bio,
we expect to yoyo around.
Looks like a buying opportunity to me.
I don't know the answer Percival;
In general, of they can make an oral formulation work, they will.
But i bet they are trying to think their way around it now!
They sell, we buy , now while it's really cheap.
We have a massive pipeline, a passel of BP partnerships,
now, all we need is patience.
Shorts must be leaking their fingers!!
I believe Goldman Sachs is Shorting this Stock!! Lets remember that the US government recently punnished Goldman Sachs for shorting and profiting during the recent recession!!
Wall-Street is full of crooks!!
Must be painful with the losses to many Longs!!
So does Golman Sach's rating mean that we gonna hit $25???
Is injection the only safe way to use whatever that treatment
is!!??
Goldman Sach's Cut to Sell did not help neither!!
Still beating us up over 5 injection site reactions. Down about 20% all told.
Can some knowledgeable I Hub member tell us if there's a technique to control injection site reactions?
Topical steroids? Multiple smaller injections? etc.
|
Followers
|
47
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
494
|
|
Created
|
02/21/06
|
Type
|
Free
|
| Moderators | |||
Ionis Pharmaceuticals, Inc., through its drug discovery platform based on antisense technology, discovers and develops drugs that bind to RNA antisense technology. The company has commercialized its antisense drug and has 19 drugs in development. Ionis' drug development programs are focused on treating cardiovascular, metabolic, and severe neurodegenerative diseases and cancer. It licenses its drugs to partners prior to late-phase development and commercialization. The company and Alnylam Pharmaceuticals jointly own Regulus Therapeutics Inc., a company focused on the discovery, development, and commercialization of microRNA therapeutics. Ionis is the owner or exclusive licensee of approximately 1,600 issued patents worldwide. The company was founded in 1989 and is based in Carlsbad, California.
| Volume: | 2,292,742 |
| Day Range: | 26.875 - 28.495 |
| Last Trade Time: | 7:20:11 PM EDT |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
