Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
$GOVX bottom bio recent r/s 2.48M float with $3.54 cash per share and warrants at $6.21 CEO and CFO bought at these levels this month + 13G of 9.99% from 14/02/24
— STOCKS Gambino (@StocksGambino) February 26, 2024
''GeoVax to Present at the 2024 BIO CEO & Investor Conference at February 26, 2024, 4:15 pm ET'' pic.twitter.com/8sXy5x4gpa
GOVX: effective Jan. 31,2024 a one for 15 reverse split:
https://hedgefollow.com/upcoming-stock-splits.php
they've been trying for weeks to pump up the price before the R/S, but it didn't fool anyone.
Interesting to see the SP here when the dust settles after the R/S.
Will average down when it hits .05 again
Merry Christmas $$$$$$$$
Are you surprised?
This is a R/S dump stock.
The only ones that benefit from holding this stock are the crafty/lucky peeps or the insiders.
How long before the next R/S makes this stock look attractive to investors that don't know any better than to look past the hype?
just went to ZERO BORROW,no shares to short,never been optionable
Going up river $$$$$$ FISH ON $$$$$
is this trash still listed? unbelievable
Three months with no fundamental change, the elastic on the jock strap is worn out.
Hold on to your jock straps $$$$$$$$
.6902 intraday gap fill this week and looking at Late Feb for next opportunity imo
I have no information on these important questions.
How soon could they do it? Do they have the money for it?
Develop the clinical/health applications for their patents, monetize these patents.
What are they expected todo next?
You failed to read the News...
it is hopeless, P&D on no news, will crash as always. Obsolete trash
Analysts calling for a minimum of $3 and a maximum of $8!!! LET'S GET IT!
H.C. Wainwright analyst Vernon Bernardino reiterated a Buy rating on GeoVax Labs and set a price target of $8.00.
Currently, the analyst consensus on GeoVax Labs is a Moderate Buy with an average price target of $5.50
In a report released yesterday, Maxim Group also maintained a Buy rating on the stock with a $3.00 price target.
Ya I've heard that, and they're over 400 different strains of the flu too...I mean Covid, you know...variants...lol
Might want to check your information. The Sudan Ebola virus is not the same as the Zaire Ebola virus. There are biological differences and as such the vaccines aren't interchangeable.
GOVX
In the works? The Ebola vaccine was already developed back in 2019'
Ebola is spreading, they have the vax in the works...nuff said
GeoVax to Host Third Quarter 2022 Business Update and Financial Results Conference Call on November 9, 2022
October 26 2022 - 09:00AM
GlobeNewswire Inc.
Alert
Print
Share On Facebook
via NewMediaWire – GeoVax Labs, Inc. (Nasdaq: GOVX), a clinical-stage biotechnology company developing immunotherapies and vaccines for cancer and infectious diseases, announced today that it will report third quarter 2022 financial results after the market close on Wednesday, November 9, 2022.
Following the release, management will host a live conference call and webcast to provide a general business update and discuss financial results. Subsequent to management’s prepared remarks, there will be a Q&A period for analysts and others.
Wednesday, November 9, 2022, 4:30 PM EST
Domestic: 877-269-7756
International: 201-689-7817
Conference ID: 13733113
Webcast: GeoVax Earnings Webcast
A webcast replay of the call will be available via the same link as the live webcast approximately one hour after the end of the call through February 9, 2023. A telephonic replay of the call can be accessed by calling 877-660-6853 (domestic) or 201-612-7415 (international), using access code 13733113. The telephonic replay will be available until November 23, 2022.
About GeoVax
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing novel therapies and vaccines for cancers and many of the world’s most threatening infectious diseases. The company’s lead program in oncology is a novel oncolytic solid tumor gene-directed therapy, Gedeptin®, presently in a multicenter Phase 1/2 clinical trial for advanced head and neck cancers. GeoVax’s lead infectious disease candidate is GEO-CM04S1, a next-generation COVID-19 vaccine targeting high-risk immunocompromised patient populations. Currently in two Phase 2 clinical trials, GEO-CM04S1 is being evaluated as a single-dose, COVID-19 vaccine for immunocompromised patients such as those suffering from hematologic cancers and other patient populations for whom the current authorized COVID-19 vaccines are insufficient. In addition, GEO-CM04S1 is in a Phase 2 clinical trial evaluating the vaccine as a more robust, durable COVID-19 booster among healthy patients who previously received the mRNA vaccines. GeoVax has a leadership team who have driven significant value creation across multiple life science companies over the past several decades. For more information, visit our website: www.geovax.com.
Media Relations Contact:
Gina Cestari
6 Degrees
917-797-7904
gcestari@6degreespr.com
This Company is not garbage. This Company consists of dedicated, hard-working human beings, with the goal of producing effective medicines for the healing and survival of sick humanity.
is this garbage still listed on nasdaq? Cannot believe it
You are welcome.
I watched the conference and it appears they have a chance of becoming a profitable biotech company.
The pipeline is quite robust, imo. Their approach of activating the T Cells stands to make their platform for vaccines be very valuable and very successful, if they can commercialize and develop their pipeline. All my opinion of course.
Thanks for sharing that link.
The Investment conference begins in 10mins.
Tune in to the webcast with this link:
https://journey.ct.events/view/883f0eb3-d86b-41c6-906b-33bbce4c006e
$GOVX
A break and hold over $1.80 sets this up nicely for $2.30-2.50. Let's get it.
http://stocktwits.com/LaDolceVita7/message/484220525
|
Followers
|
260
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
19459
|
|
Created
|
12/11/01
|
Type
|
Free
|
| Moderators | |||
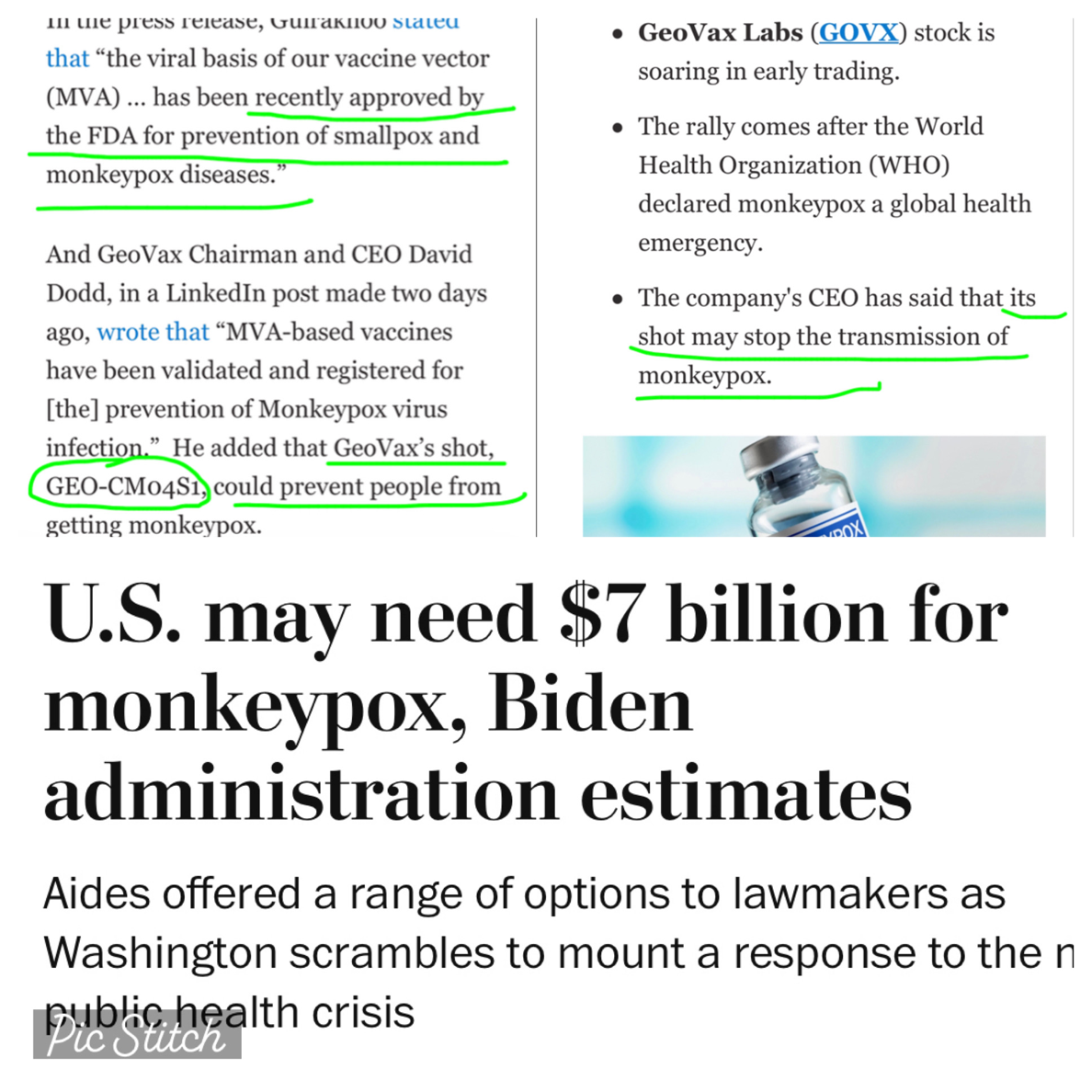
July 26 (Reuters) - The Biden administration estimates it may need nearly $7 billion to deal with the monkeypox outbreak in the United States, the Washington Post reported on Tuesday.
The funding estimate was part of a memo marked to President Joe Biden outlining a series of options, as opposed to a formal funding request, the report said. (https://wapo.st/3oxXimZ)
The White House and the U.S. Department of Health and Human Services did not immediately respond to requests for comment.
CREDIT MONEYMADE FOR DD AND ALERTING GROUP BEFORE THE CLOSE. WEEEEEEEE $1.70https://investorshub.advfn.com/boards/read_msg.aspx?message_id=169502961
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
