Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
The 5 analysts offering 12-month price forecasts for Delcath Systems Inc have a median target of 18.00, with a high estimate of 22.00 and a low estimate of 13.00. The median estimate represents a +216.62% increase from the last price of 5.69.
H.C. Wainwright Reaffirms Their Buy Rating on Delcath Systems (DCTH)
TipRanks
Aug. 15, 2023, 09:35 AM
In a report released today, Swayampakula Ramakanth from H.C. Wainwright maintained a Buy rating on Delcath Systems (DCTH – Research Report), with a price target of $18.00. The company’s shares opened today at $5.84.
HC Wainwright & Co. Maintains Delcath Systems (DCTH) Buy Recommendation
Story by George Maybach • 14h
?
Fintel reports that on August 15, 2023, HC Wainwright & Co. maintained coverage of Delcath Systems (NASDAQ:DCTH) with a Buy recommendation.
Analyst Price Forecast Suggests 436.15% Upside
As of August 2, 2023, the average one-year price target for Delcath Systems is 16.73. The forecasts range from a low of 10.10 to a high of $23.10. The average price target represents an increase of 436.15% from its latest reported closing price of 3.12.
Hope you get that back working soon. I use TD Ameritrade and so far haven't had any problems with their system.
No... On my app and my laptop I have a message saying they are unable to at the moment
$DCTH
Wow, bummer. Did they say why?
Fidelity is down. Cant make trades at all.
$DCTH
Awesome! I need to get my correct my sleeping habits - kicking myself today.
Ive been able to do 5.01 to 5.77 and 5.55 to 7.25. In again at 6.33.
Nice move CD. Still climbing. $$DCTH$$
I'm in at 5.43, went back to bed and missed the flip today. Oh well might see 10 by EOW.
This board needs some life!! Lets go $DCTH
round ii
filled as low as 6.46 on that DIP
$DCTH
$7!!!!!!!!!!!!!!
SOLD SOME 6.95 AFTER 7 COULDNT BREAK HARD
Told you guys. Get in now before it's too late.
Wait until it cross $6. You will see $10 today or tomorrow.
6.50 WAS A NICE POP OFF MY 5.55 ENTRY
Yeah there's resistance at that level, but I believe it'll break through today. Too much buying pressure here. FDA approval is huge news.
6.24 SO FAR BUT THEY QUICKLY SOLD IT OFF TO 5.34
DCTH
Good morning. I wonder how high this flies today.
https://finviz.com/quote.ashx?t=DCTH&p=d
$DCTH 🚨 Approved
— Ripster🪙📈📉 (@ripster47) August 15, 2023
Finally after years of try.
Delcath Systems, Inc. Announces FDA Approval of HEPZATO KIT™ for the Treatment of Adult Patients with Unresectable Hepatic-Dominant Metastatic Uveal Melanoma. pic.twitter.com/ZCBQZXbgzE
Where is everybody?
Wow stock is down 2 weeks before PDUFA date. Hard to imagine this wont be approved this time with no adcom. Not sure why there arent more buyers. Hopefully there is a pop on approval and they have a quick launch and revenue ramp.
Finally looks promising for real. Had shares few years ago and got chewed up and spit out . Reverse splits wiped out a lot of investors. Looks like they turned the corner and could become the investment everyone hoped for awhile back. Good luck to those holding.
No one loving the start of the run?
A new corporate presentation was posted to the company's website with alot of updated information.
https://delcath.com/investors/events-presentations/
This company has been left for dead by the market but I think the stock could recover strongly next year after approval if revenues begin to roll in. I would be interested to hear others thoughts on catalysts and stock price targets. I think at least a $100 million market cap should be expected on approval. Longer term at least $250 million market cap when revenues are material. Starting 3 new trials in 2023 will be positive as well. There appears to be little interest or attention for the company now but hopefully that changes after approval. My biggest fear is that they will have to dilute more at these awful rock bottom prices.
just waiting.........................................................................
yup--- good chat with GA... TY. Neal
FDA submission Summer/Fall 2022
FDA approval possible within 6 mos of submission
Fund raise? when?
Stock price lousy so wait for the fund raise?
All results on trials and use are good!
TY, Neal
Yeah--- I had Covid so I have been delayed!!! :)
|
Followers
|
497
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
50157
|
|
Created
|
05/11/05
|
Type
|
Free
|
| Moderators | |||
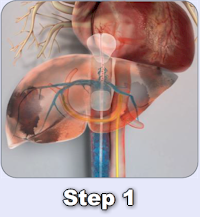
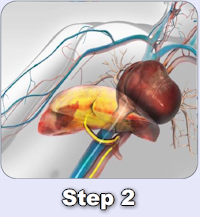
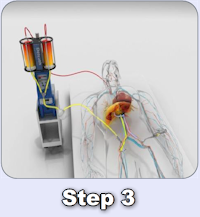


In December 2018, Delcath entered into a License Agreement with medac GmbH for the commercialization of the CHEMOSAT® system in Europe. Under the terms of the medac License, medac has the exclusive right to sell and market CHEMOSAT® in all member states of the EU, Norway, Liechtenstein, Switzerland, and the United Kingdom.
Since launching CHEMOSAT® in Europe, over 750 commercial treatments have been performed at over 25 European cancer centers. Physicians in Europe have used CHEMOSAT® to treat patients with a variety of cancers in the liver, primarily ocular melanoma liver metastases, and other tumor types including cutaneous melanoma, hepatocellular carcinoma, cholangiocarcinoma, and liver metastases from colorectal cancer, breast, pancreatic and neuroendocrine.

| Split # | Date | Ratio |
| 1 | April 9, 2014 | 1:16 |
| 2 | July 21, 2016 | 1:16 |
| 3 | November 6, 2017 | 1:350 |
| 4 | May 2, 2018 | 1:500 |
| 5 | December 24, 2019 | 1:700 |
| Common Shares Authorized: | 1,000,000,000 |
| Common Shares Outstanding: | 70,056 |
| Preferred Shares Authorized: | 10,000,000 |
| Preferred Shares Outstanding: | 41,447 |
| Warrants: | 29 | ($42; exp 2/2020 - 10/2021) |
| 2019 Warrants: | 1,826,579 | ($23.04; exp 12/2024) |
| Options: | 1,640 | |
| Total Warrants/Options: | 1,828,248 |
| Preferred Stock: | 1,799,093 |
| Convertible Notes: | 63,493 |
| Total Reserved: | 1,862,586 |
| Jennifer K. Simpson, Ph.D. | : 2,965 |
| John Purpura, M.S. | : 2,830 |
| William D. Rueckert | : 2,619 |
| Roger G. Stoll, Ph.D. | : 4,047 |
| Marco Taglietti, M.D. | : 2,606 |
| Total Shares | : 21,459 |
| Altium Capital Management L.P. | : 7,204 |
| Rosalind Master Fund L.P. | : 7,024 |
| Hudson Bay Master Fund Ltd. | : 7,024 |
| Total Shares | : 21,612 |
| 45 Shareholders (est.) | : 26,985 |
| Company Website: Delcath Systems, Inc. | Stock and Financial Website: OTC Marketplace |
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
