Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
Gets out now millions of offering shares at .69…12,325,000 common shares. Each common share was sold at an offering price of $0.69 per share, for gross proceeds of approximately $8.5 million,
BriaCell Announces FDA-Authorized Expanded Access Policy for Metastatic Breast Cancer Patients
Sorry. Wrong Board.
aks yourself why no BP wanted to be a part of this. Plenty of time to notice?
BCTX..........................https://stockcharts.com/h-sc/ui?s=BCTX&p=W&b=5&g=0&id=p86431144783
I'm predicting an offering this week... Company needs cash. Under $1m currently
BriaCell Reports Positive Overall Survival (OS) in Metastatic Breast Cancer
Median overall survival of 15.6 months in Phase 2 Bria-IMT™ study patients treated in combination with immune checkpoint inhibitor
OS of 15.6 months compares favorably with 6.7-9.3 months reported for similar patients in the literature
Ongoing Phase 3 study investigating Bria-IMT™ in similar metastatic breast cancer population
No drug related discontinuations to date
PHILADELPHIA and VANCOUVER, British Columbia, Sept. 11, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to announce positive overall survival data of its Phase 2 clinical study of Bria-IMT™ in combination with an immune check point inhibitor (CPI) in late stage metastatic breast cancer.
https://www.otcmarkets.com/stock/BCTX/news/story?e&id=2978177
BriaCell Reports Positive Overall Survival (OS) in Metastatic Breast Cancer
Good news and it goes lower again,,,lol
$BCTX Shows “Strong Anti-Cancer Activity of Bria-OTS+™ and Bria-PROS+™ Clinical Candidates for Breast and Prostate Cancer”
Press Release: https://bit.ly/4aJmAoc
$BCTX #CancerTreatment #Innovation
Does anyone have information about the stock spinoff that occurred last year with this company? I have 600 shares of the spinoff security with 0 value and has remained that way since the spinoff. Thanks…
$BCTX is thrilled to announce a new remarkable responder in our Phase 2 study of the Bria-IMT™ combination regimen. https://finance.yahoo.com/news/briacell-records-responder-remarkable-improvement-120000404.html
Good news today. I took a entry
2023 Anticipated Milestones, please?
$BCTX $15 Price Target
Zacks Small-Cap Research just released a new report on BriaCell Therapeutics Corp. (Nasdaq: BCTX) with a $15 price target.
Read the full report here: https://scr.zacks.com/.../BCTX-Posters.../default.aspx
#Stocks #StocksToWatch #StockMarket
BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) was featured in an article on Seeking Alpha
Discover more about this #BioTech company here: https://bit.ly/3DoEbUc
BriaCell Therapeutics: Fast-Tracking High-Potential Breast Cancer Immunotherapy
#Stocks #StocksToWatch
Strange stock, but I've loved having it the past couple of years. I've mostly day traded, as there was a pretty good pattern last year, of early drops and late charges, and vice versa. I did end up getting stuck at around $10 so have been holding a lot at that level. Hopefully it runs again and I can keep chipping away at my mortgage because of the fancy researchers in Vancouver. Best of luck, all!
Yep, I'm still here. She's getting ready to burst! Low float/high short interest, Fast track approval and now up on big volume. Gonna get interesting!
BriaCell Therapeutics Price Target Announced at $25.00/Share by HC Wainwright & Co.
BriaCell Reports Received FDA Fast Track Approval For Its Targeted Breast Cancer Immunotherapy
BCTX
I'm still here....wait til you see what happens this week
We will dance!
Closed above resistance of $10.50....We dance on Monday
Low float/high short interest. Setting up for big run
$BCTX BriaCell is an immuno-oncology focused biotechnology company developing targeted and effective approaches for the management of cancer. More information is available at https://briacell.com/.
$BCTX announces the addition of Suzanne Ostrand-Rosenberg, Ph.D. to its Scientific Advisory Board.
Dr. Ostrand-Rosenberg has more than 40 years of experience leading investigations focused on the immune system’s response to cancer. She is currently the Robert & Jane Meyerhoff Professor of Biochemistry, Emeritus, and Professor of Biological Sciences, Emeritus, University of Maryland Baltimore County, Baltimore, MD, and has been working with the University since 1977. She was also appointed as the Adjunct Professor of Pathology, Huntsman Cancer Institute (HCI), University of Utah, Salt Lake City, UT, in 2018. https://finance.yahoo.com/news/briacell-adds-pioneering-immunologist-cancer-130000276.html
$BCTX has added Pioneering Immunologist and Cancer Immunotherapy Expert, Suzanne Ostrand-Rosenberg, Ph.D., to its Scientific Advisory Board!
Click here for a bio and to learn what she's bringing to the BriaCell team!
https://yhoo.it/3kbmmhX
Easy Money for more $ZOM shares $BCTX thanks to @MrZackMorris for the tip in his Reddit community.. called this out last month on my Instagram big profits.. shorts are still all over this.. squeeze is to be prepared for.. get in profit big and get out .. October is #BreastCancerAwarenessMonth also load on $ZOM ?? it acquired Pulse Vet
$BCTX thanks to @MrZackMorris for the tip in his Reddit community.. called this out last month on my Instagram big profits.. shorts are all over this now.. squeeze is to be prepared for.. get in profit big and get out .. October is #BreastCancerAwarenessMonth also load on $ZOM 💎 pic.twitter.com/wJVS4lZydq
— BL3 (@SellingStrikes) October 3, 2021
(Nasdaq: BCTX, BCTXW) (TSX-V: BCT) CEO highlights key achievements of the company including recent collaboration with ImaginAb https://yhoo.it/3CDPzc7
BriaCell Therapeutics Corp. (Nasdaq: $BCTX, $BCTXW) (TSX-V:BCT) News Alert https://yhoo.it/3gmjQ6A #stocks #medical #stockstowatch
MarketsandMarkets 4th Annual Next Gen Immuno-Oncology Virtual Congress-US Edition: June 28-30, 2021
Dr. Williams will be speaking at the scientific conference at 11:30am ET on June 29, 2021.
The topic is “Personalized, off-the-shelf cellular immunotherapy for cancer”.
A copy of the presentation will be posted here: https://briacell.com/novel-technology/scientific-publications/.
For additional information on the conference and the speaker, please visit:
https://events.marketsandmarkets.com/4th-annual-next-gen-immuno-oncology-virtual-congress-us-edition/speakers
Separately, BriaCell has recently appointed Miguel A. Lopez-Lago, Ph.D. as Senior Director, Research and Development. Since 2000, Dr. Lopez-Lago has been working as a cancer scientist at Memorial Sloan-Kettering Cancer Center, New York (MSKCC). Specifically, he has investigated various aspects of tumor biology, including the development of targeted therapies for Mesothelioma and the characterization of the biological mechanisms underlying cancer metastasis. More recently, Dr. Lopez-Lago has been interested in the study of the tumor immune-microenvironment and in the development of immunotherapies for thoracic cancers using chimeric antigen receptor (CAR) T cell technologies. Since 2013, Dr. Lopez-Lago has been working as Senior Research Scientist at MSKCC. Dr. Lopez-Lago received his Bachelor of Science in Bio-Sciences and his doctorate in Molecular Biology from Santiago of Compostela University, Spain.
About BriaCell Therapeutics Corp.
BriaCell is an immuno-oncology focused biotechnology company developing targeted and effective approaches for the management of cancer.
For additional information on BriaCell, please visit: https://briacell.com/.
|
Followers
|
24
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
349
|
|
Created
|
03/14/15
|
Type
|
Free
|
| Moderators | |||
Dedicated to enhance the lives of cancer patients who are facing limited therapeutic options, BriaCell Therapeutics Corp. (OTCQB: BCTXF; TSX: BCT.V)’s mission has been to develop novel immunotherapies, as the most cutting edge technology to fight cancer. Immunotherapies have become the forefront of the cancer treatments because they use the body’s immune system to destroy the cancer cells, offering higher levels of safety and efficacy than chemotherapy, with less likeliness of recurrence.
Designed by our team of scientists and clinicians, BriaCell’s proprietary whole-cell based vaccine technology platform continues to show its impressive potential to establish a new model for treating cancer patients. Our lead product candidate, Bria-IMT™ (SV-BR-1-GM), is a genetically engineered whole-cell vaccine derived from a human breast cancer cell line. Bria-IMT™ is used in combination with multiple immune-modulators to powerfully trigger the immune system to recognize and eliminate cancerous cells.
In a preliminary Phase I clinical study in metastatic (i.e., Stage IV) breast cancer patients who had failed multiple treatments, Bria-IMT™ treatment significantly reduced the tumor size, without serious side effects. Importantly, the tumor regression was observed in other sites including the lung and even the brain -a difficult site. Impressively, the median lifespan of the patients was substantially longer than that of the comparable trials.
At our laboratory facility in Berkeley, CA, we are planning to expand our pipeline of oncology immunotherapy candidates using our proprietary technology platform.
Using the clinical data from the patients from the clinical studies, we are working to identify the molecular fingerprint of the patients for which the vaccine would be highly effective, and are planning to develop diagnostic testing products to identify this group. By directing the drug to the top-responders, we expect this approach to increase the likelihood of clinical trial success of Bria-IMT™ -to bring hope to thousands of cancer patients with few-to-no treatment options.
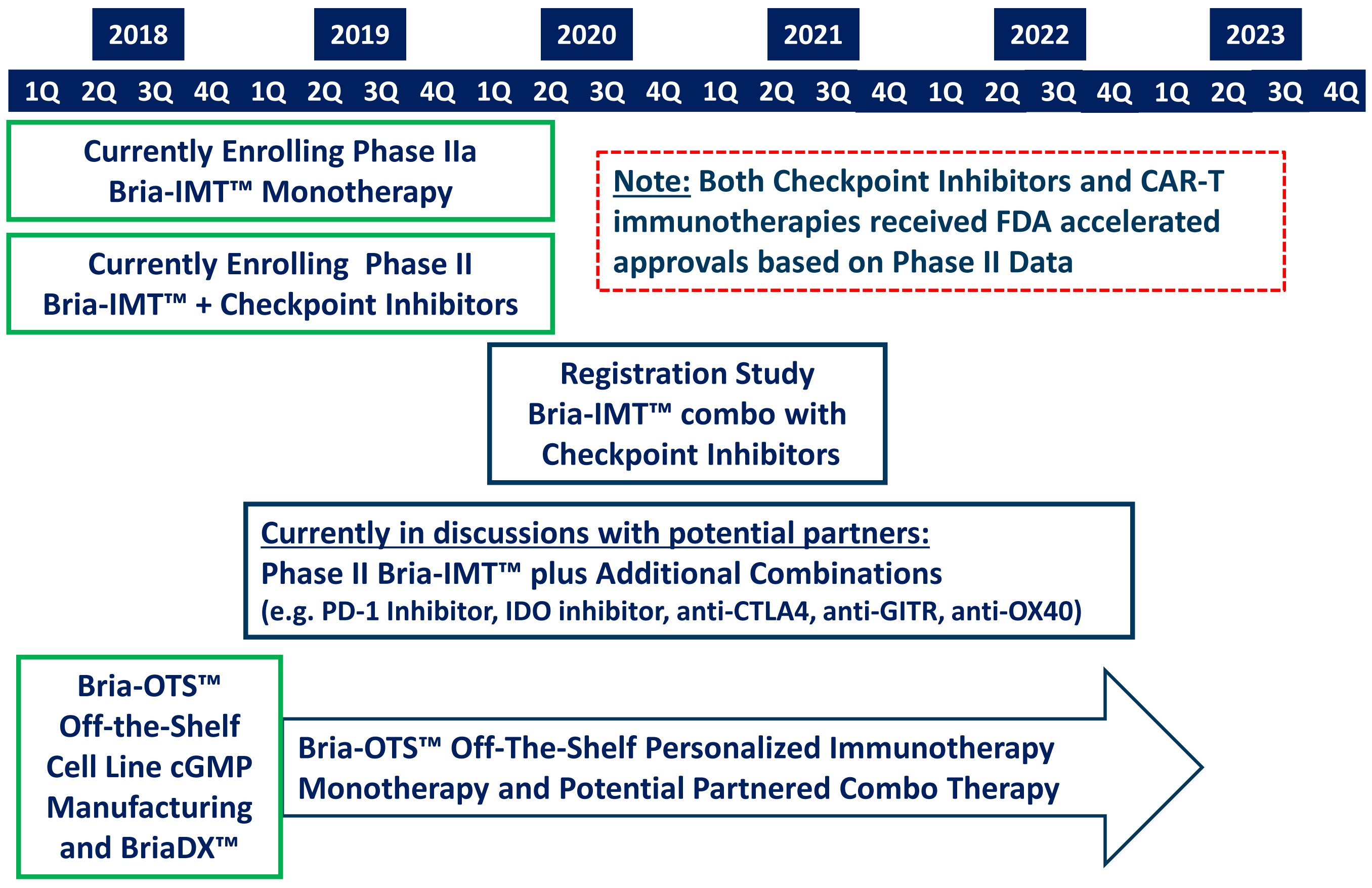
The company is currently recruiting its Phase I/IIa trial to further test Bria-IMT™ safety and activity in metastatic breast cancer patients. BriaCell maintains global rights for Bria-IMT™.
BriaCell is currently recruiting patients to evaluate the safety and activity of Bria-IMT™ in an additional Phase I/IIa study in 25-40 metastatic breast cancer patients who have failed at least one course of treatment.
For the details of the trial, please visit For the details of the trial, please visit https://www.clinicaltrials.gov/ct2/show/NCT03066947
FDA has approved the roll-over combination study of Bria-IMT™ with pembrolizumab (Keytruda; manufactured by Merck & Co., Inc.) or ipilimumab (Yervoy; manufactured by Bristol-Myers Squibb Company) for patients previously treated with BriaVax™ from the ongoing Phase I/IIa Clinical Trial in Advanced Breast Cancer. The study is available for patents on the SV-BR-1-GM Phase I/IIa study who develop progressive disease. These patients will be eligible to roll-over into combination therapy with Keytruda or Yervoy, depending on the type of breast cancer they have.
More information on the roll-over combination study of Bria-IMT™ with either ipilimumab or pembrolizumab will be available on ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03328026)
Manufactured by Merck & Co., Inc., KEYTRUDA® (pembrolizumab) is a prescription medicine that may treat certain cancers by working with the immune system. It has been approved for the treatment of a number of cancer indications excluding breast cancer.
For more information on pembrolizumab, please see:
http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
Manufactured by Bristol-Myers Squibb Company, YERVOY® (ipilimumab) is a prescription medicine used in adults and children 12 years and older to treat melanoma (a kind of skin cancer) that has spread (metastatic) or cannot be removed by surgery (unresectable). It is a monoclonal antibody that works to activate the immune system and enabling them to recognize and destroy cancer cells.
For more informations on Ipilimumab, please see:
https://packageinserts.bms.com/pi/pi_yervoy.pdf
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
