Register for free to join our community of investors and share your ideas. You will also get access to streaming quotes, interactive charts, trades, portfolio, live options flow and more tools.
ADAP In at $1.14
Read the 10Q. Great numbers all around.
Big 'tute presence.
Trading not far from cash value
FDA Approval 8/01/24
Plenty of head room
Lotta shares slowing the move, but direction is up.
http://archive.fast-edgar.com/20240812/AQZZ762CZC22UZB2222Q2ZZ27S39ZM2IHN62
$ADAP Nice Q!
(in thousands)
2024 ......... 2023
Net profit/(loss)
$69,521...... $(21,389)
$70M profit, from $21M loss, YoY!
just give it some time prior to the PDUFA. I dont have a position and i dont need to play.
Id only buy some if there is strength....
I don't think this does a run up if that's what you are looking for. Downtrend is strong here.
This is a pure momo play-- the science is not so convincing.
Think itll cover some ground before PDUFA
Adcs as the future of solid tumors? Ate you serious?
Genetech was doing some corporate wide cost cutting, so it's hard to tell. The galapogos deal is pretty decent. I like that they have skin in the game with their mfg tech. I think it is a decent buy in this range it's been stuck at.
ADAP.................https://stockcharts.com/h-sc/ui?s=ADAP&p=W&b=5&g=0&id=p86431144783
ADC is the future for solid tumors, no wait time, decent ORR. Why bother with TCR-T and deal with matching HLA, long wait and PD1 for persistence. All the CARNK bios are pivoting to SLE ....Will ADAP try a TCR-T + mAb combo or TCT-T + CBNK to improve ORR? Can 1 accountant innovate?
The question remains, was it due to a refocused R&D strategy or data driven.
Upcoming Investor Day (Apr 18, 8:00 am EDT) https://adaptimmune.zoom.us/webinar/register/WN_SAFMQyw3QbiyM9wSLIaTqw#/registration
The multi-year strategic collaboration they struck with RHHBY's Genentech has been terminated. The agreement, established initially for eight years, aimed at launching cell therapies for various cancer indications using ADAP's iPSC platform. Per the terms, ADAP received $150M upfront and was expected to receive $150M in additional payments over five years. ADAP was also eligible to receive development and other milestone-based payments totalling as much as $3B.
https://finance.yahoo.com/news/strategic-collaboration-between-adaptimmune-genentech-120000529.html
There is going to be clinical data from LYEL, CRSP and Arsenal Bio* over the next few years that could show if T-cell ''exhaustion'' can be overcome.
* The synthetic pathway activators that Arsenal Bio have created help with maintenance of T-cell stem-like phenotypes, and restrict accessibility of various exhaustion marker genes
ADAP.......................................................................https://stockcharts.com/h-sc/ui?s=ADAP&p=W&b=5&g=0&id=p86431144783
ADAP.............................................https://stockcharts.com/h-sc/ui?s=ADAP&p=W&b=5&g=0&id=p86431144783
This explains why TIL, TCR-T, Tigit, ... not working. Exhausted T is a misnomer
https://www.cell.com/cell-reports/fulltext/S2211-1247(24)00040-8?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124724000408%3Fshowall%3Dtrue
WOOOOW 27000 SHARE BUY $$$$$$$$$$$
Adaptimmune Announces U.S. FDA Acceptance of Biologics License Application for Afami-cel for the Treatment of Advanced Synovial Sarcoma with Priority Review
Newsfile Corp.
Newsfile Corp
If approved, afami-cel will be the first engineered T-cell therapy for solid tumors and the first effective treatment option for synovial sarcoma in more than a decade
Philadelphia, Pennsylvania and Oxford, United Kingdom--(Newsfile Corp. - January 31, 2024) - Adaptimmune Therapeutics plc (NASDAQ: ADAP), a company redefining the treatment of solid tumor cancers with cell therapy, today announced that the U.S. Food and Drug Administration (FDA) has accepted for priority review its Biologics License Application (BLA) for afami-cel, an investigational engineered T-cell therapy for advanced synovial sarcoma. The application has a Prescription Drug User Fee Act (PDUFA) target action date of August 4, 2024.
Adrian Rawcliffe, Adaptimmune's Chief Executive Officer: “The FDA’s acceptance of the BLA submission brings us one step closer to redefining treatment for people with synovial sarcoma. Our franchise has great potential and, if approved, we have the capabilities and the capital to launch afami-cel - the first engineered T-cell therapy on the market for a solid tumor cancer.”
Dennis Williams, PharmD, Senior VP of Late-Stage Development: "Historic outcomes are poor for advanced synovial sarcoma, with low objective response rates for second-line therapies and overall survival of less than 12 months for people who have received two or more prior lines of therapy. In clinical trials, afami-cel has demonstrated an impressive response rate of ~39% among heavily pre-treated patients with advanced synovial sarcoma and about a 17-month median survival. This regulatory milestone is a testament to our teams' relentless work to deliver a novel treatment option to more people diagnosed with synovial sarcoma."
This acceptance is supported by positive data from Cohort 1 of the pivotal trial SPEARHEAD-1, which met its primary endpoint for efficacy. Data from the trial were presented at the Connective Tissue Oncology Society (CTOS) 2023 Annual Meeting.
when was the last time the mgt. didn't missed a milestone promised. BLA, PRAME, ...
Hard to say, but they have only talked about sequential/combination treatment with afami-cel and lete-cel, not committed to it. Based on what others are doing, I would like them to pick up the pass of innovation. Based on their own data, a TGF-B signature was measured across tumour indications, so think they should test the combination of NY-ESO-1 with MAGE-A4 TCRs that co-express the CD8a co-receptor with a dnTGF-BRII as well. They also ran a sub-study testing afami-cel with low-dose radiation. While small, it did show greater detection of SPEAR T-cells in tumour biopsies when infusion followed it. As for TC-520 (targeting CD70), I think it should be dropped.
May be the investors realized that CART, CARNK targeting single antigen not going to subdue solid cancers. Will 2nd gen SPEAR cells NY-ESO + MAGE-A4 combo or extracellular CD70 + SPEAR T cells combo avoid the CAR crash?
Has the CEO finally awoken?!
''Sarcoma franchise of afami-cel and lete-cel leverages same development and commercial footprint with US peak year sales projected to be up to $400 million
BLA submitted in 2023 for afami-cel; projected acceptance by US FDA in Q1 2024
Company preparing for US launch of afami-cel in 2H 2024 upon expected approval
Pivotal trial for lete-cel met its primary endpoint for efficacy; full data set
in Q3 2024
Company working toward approval of lete-cel; planning for US commercial launch in 2026
Company funded into early 2026 with > $300 million including existing balance sheet, projected payments from partners, and other non-dilutive capital sources'' https://finance.yahoo.com/news/adaptimmune-projects-sarcoma-franchise-deliver-123700704.html
CARTs, ADCs not investable. Too many to count. Targeted bombing offers no OS benefits but long term health issues, secondary cancers and infections.
Hard to say, but in the next few I expect to see datasets from others trials (testing ADCs) in platinum-resistant ovarian. However, there are downsides including, TEAEs, such as those leading to drug discontinuation, interruption or reduction. Also, dosing every 'x' amount of weeks.
Very nice read
Thanks for posting
I wonder why they no longer talk about overexpression of PDE4C or PDE7A (the latter increases the ability to induce apoptosis in target cells, confers (partial) resistance to the inhibitory effects of forskolin, PGE2 and adenosine, as well as (partially) resistance to the inhibition of cytokine release by mediators that increase intracellular cAMP). Also, inducible IL-7 and inducible IL-15
https://www.biospace.com/article/releases/adaptimmune-selects-adp-600-as-clinical-candidate-for-best-in-class-prame-strategy/
They should have taken a next-gen version* into the clinic instead. This is based on where IMTX is and the data generated to date https://investors.immatics.com/static-files/138d7b76-d252-4c61-a824-e17307c4f537
* I think it should include a CD8a co-receptor and dominant-negative TGF-B receptor https://journals.aai.org/jimmunol/article/208/1/169/234163/Engineering-Cancer-Antigen-Specific-T-Cells-to
New NY-ESO-1 data https://ctos2023.eventscribe.net/index.asp?posterTarget=604312
This is not good enough to survive the ADC tsunami.
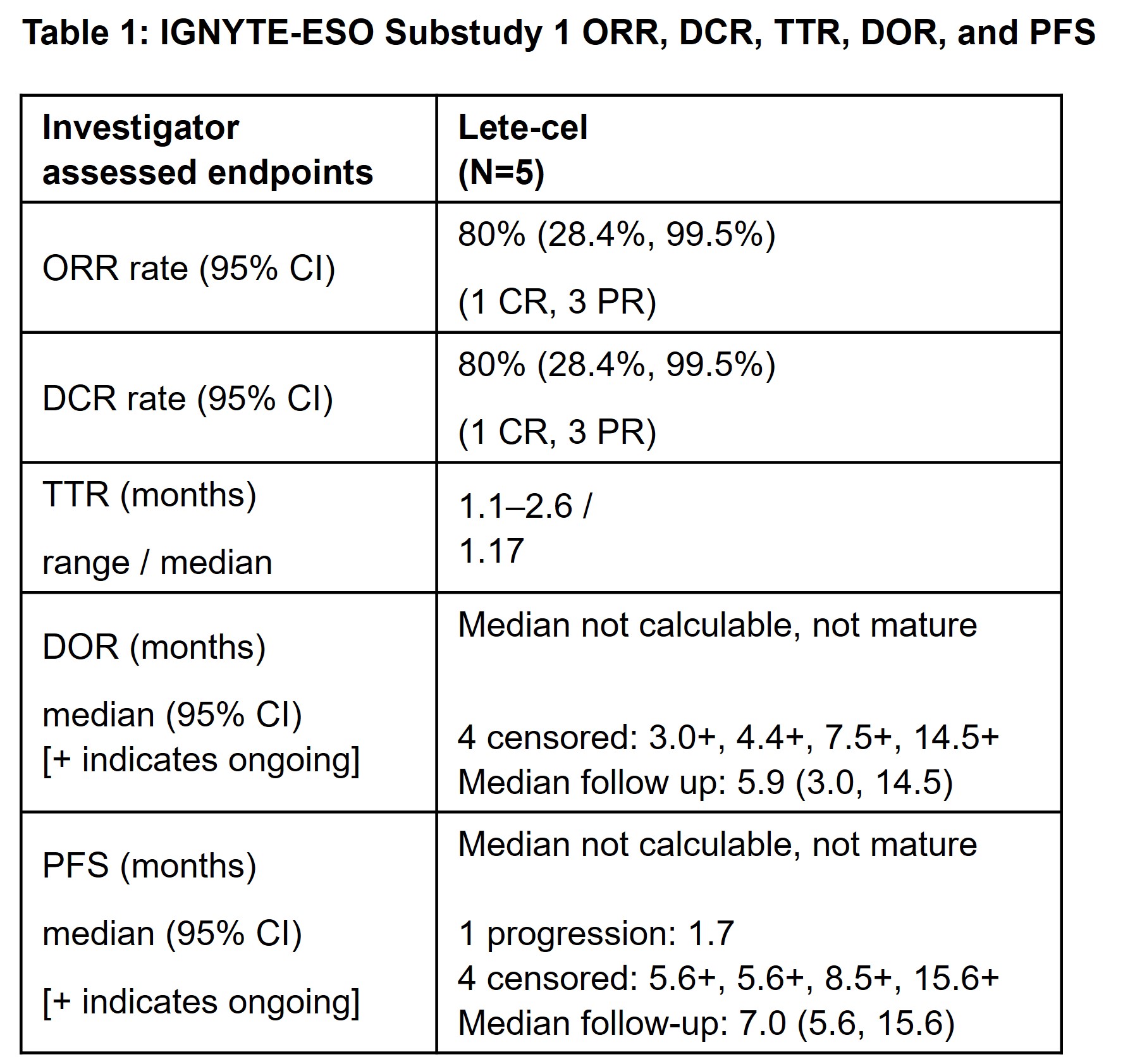
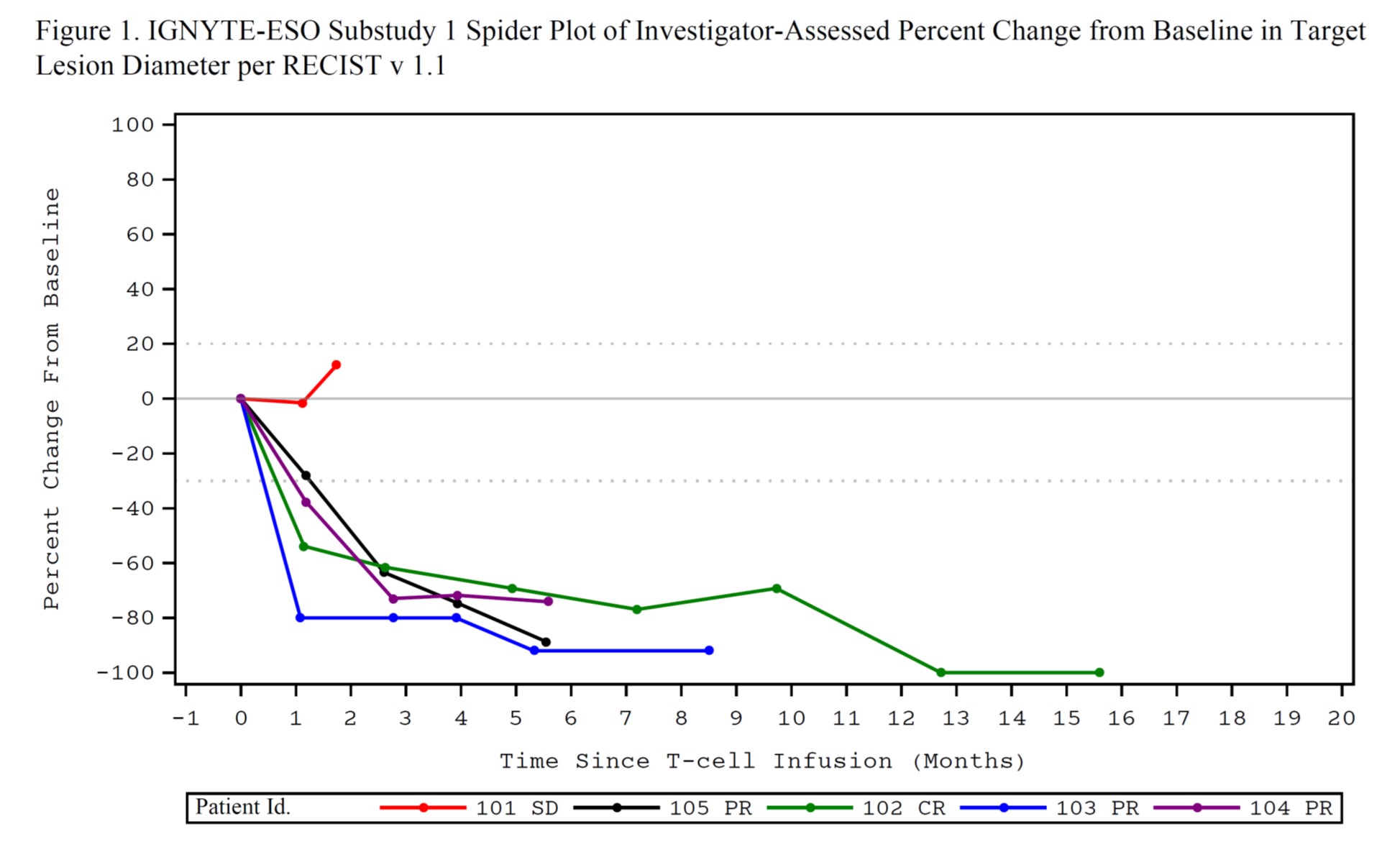
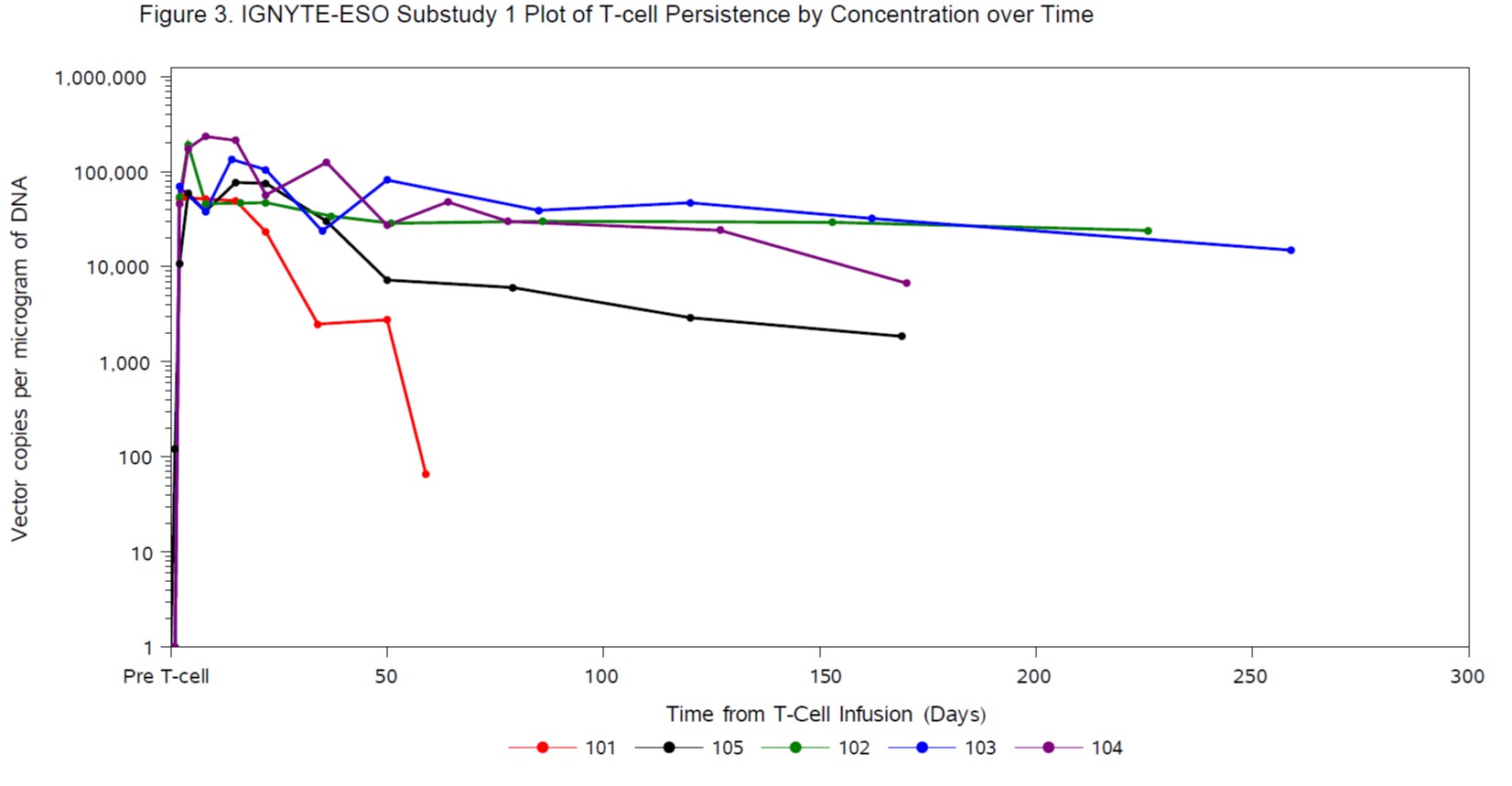
35.6% (2 complete response, 14 partial response [PR]). Median duration of response was 20.79 weeks (95%CI: 11.6, 30.9)
The ESMO abstract
1019O - Clinical and translational data from the phase I SURPASS trial of ADP-A2M4CD8 T cell receptor (TCR) T cell therapy alone or combined with nivolumab in solid tumors
Background
ADP-A2M4CD8 is a TCR T-cell therapy targeting melanoma-associated antigen A4 (MAGE-A4), modified with a CD8a coreceptor, to treat human leukocyte antigen A*02–eligible patients (pts) with unresectable/metastatic solid tumors. ADP-A2M4CD8 monotherapy has demonstrated an acceptable benefit-to-risk profile and encouraging anti-tumor activity in the ongoing phase 1 SURPASS trial. Nevertheless, inhibition of immunosuppressive pathways may improve anti-tumor responses; therefore, new SURPASS cohorts include ADP-A2M4CD8 combined with nivolumab or pembrolizumab. We report data from pts receiving monotherapy and nivolumab combination therapy.
Methods
T-cells are obtained by leukapheresis, transduced with a lentiviral vector carrying the TCR and CD8a coreceptor genes, expanded ex vivo, and infused back to the pt following lymphodepleting chemotherapy. ~Four weeks after ADP-A2M4CD8 infusion, a subset of pts also received nivolumab 480 mg every 4 weeks until unacceptable toxicity/disease progression. Primary and secondary objectives are safety and anti-tumor activity, respectively.
Results
As of March 9, 2023, 51 pts with a median age of 60 years (range: 31–75) mainly with melanoma, non-small cell lung, ovarian, gastroesophageal, head and neck, or urothelial cancers received 1.02–9.95x109 ADP-A2M4CD8 T-cells. Median (range) number of prior lines of therapy was 3 (1–8) and MAGE-A4 expression H-score was 250 (90–300). The four most common adverse events related to T-cell therapy were cytokine release syndrome (n=38 [74.5%]; 7 [13.7%] were Grade≥3), neutropenia (27.5%), pyrexia and fatigue (each 21.6%). Overall response rate per RECIST v1.1 by investigator review in monotherapy pts (n=45) was 35.6% (2 complete response, 14 partial response [PR]). Median duration of response was 20.79 weeks (95%CI: 11.6, 30.9). In pts assigned to nivolumab combination (n=6), there was 1 PR, 3 stable disease, and 2 not evaluable (2 pts have not received nivolumab and have no post-baseline RECIST assessment). Data from additional pts treated by August 2023 will be presented.
Conclusions
ADP-A2M4CD8 continues to show an acceptable benefit-to-risk profile, including in pts receiving nivolumab combination therapy.
|
Followers
|
25
|
Posters
|
|
|
Posts (Today)
|
0
|
Posts (Total)
|
262
|
|
Created
|
10/12/15
|
Type
|
Free
|
| Moderators | |||
| Volume | |
| Day Range: | |
| Bid Price | |
| Ask Price | |
| Last Trade Time: |
