Monday, January 06, 2014 9:12:26 PM
Note: 12-30-13 announcement of init. of Ph3 pivotal "SUNRISE" (BAVI+Doxy, n=600, > 100 sites) trial shown at end...
1-6-14: Peregrine Pharmaceuticals Receives FDA Fast Track Designation for Its Immunotherapy Bavituximab as a Potential Treatment of Second-Line Non-Small Cell Lung Cancer
· SUNRISE Pivotal Phase III Trial of Bavituximab in Second-Line NSCLC Underway
TUSTIN, Jan 6, 2014: Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM), today announced that the company has received Fast Track designation by the U.S. Food and Drug Administration (FDA) for its lead investigational immunotherapy bavituximab for the potential treatment of second-line non-small cell lung cancer (NSCLC). Recently, the company initiated SUNRISE, a pivotal Phase III clinical trial comparing bavituximab plus the chemotherapy docetaxel against placebo plus docetaxel in this indication.
"The fast track designation is a milestone for the SUNRISE trial program and represents a step closer to bringing bavituximab to the market," said Robert Garnick, Ph.D., head of regulatory affairs at Peregrine. "We are very pleased that the FDA has recognized the potential of this novel therapy as a treatment for this serious and devastating type of cancer and look forward to working closely with them to ensure the most efficient review process."
The Fast Track programs of the FDA are designed to facilitate the development and expedite the review of new drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs. Fast track status enables a sponsor to have more frequent and timely communication and meetings with the FDA regarding product development plans and may also result in eligibility for priority review of New Drug Applications. Fast track designation does not apply to a product alone but a combination of a product and specific indication.
SUNRISE ("Stimulating ImmUne RespoNse thRough BavItuximab in a PhaSE III Lung Cancer Study") is a Phase III, global, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the safety, tolerability and efficacy of bavituximab plus docetaxel in patients with second-line non-small cell lung cancer (NSCLC). The trial is evaluating bavituximab plus docetaxel versus docetaxel plus placebo in approximately 600 patients at more than 100 clinical sites worldwide. Patients with Stage IIIb/IV non-squamous, NSCLC who have progressed after standard front-line treatment are eligible for enrollment. Patients will be randomized into 1 of 2 treatment arms. All patients will receive up to six 21-day cycles of docetaxel (75 mg/m2) plus weekly infusions of either bavituximab (3mg/kg) or placebo until progression or toxicity. The primary endpoint of the trial will be overall survival. For additional information about the SUNRISE trial please visit http://www.sunrisetrial.com or ClinicalTrials.gov using Identifier NCT01999673. [ http://www.clinicaltrials.gov/ct2/show/NCT01999673 ]
About Bavituximab: A Targeted Immunotherapy
Bavituximab is a first-in-class phosphatidylserine (PS)-targeting monoclonal antibody that represents a new approach to treating cancer. PS is a highly immunosuppressive molecule usually located inside the membrane of healthy cells, but "flips" and becomes exposed on the outside of cells that line tumor blood vessels, creating a specific target for anti-cancer treatments. PS-targeting antibodies target and bind to PS and block this immunosuppressive signal, thereby enabling the immune system to recognize and fight the tumor. These data detailing the immune-stimulatory mechanism of action of PS-targeting antibodies, such as the company's lead drug candidate bavituximab, are the subject of a manuscript published in the October 2013 issue of the American Association for Cancer Research (AACR) peer-reviewed journal, Cancer Immunology Research [Pub. Online 8-19-13 http://tinyurl.com/mhjftka ]. Bavituximab is currently being evaluated in several solid tumor indications, including non-small cell lung cancer, breast cancer, liver cancer and rectal cancer with a trial in advanced melanoma anticipated to initiate in the near future.
About Peregrine Pharmaceuticals, Inc.
Peregrine Pharmaceuticals, Inc. is a biopharmaceutical company with a portfolio of innovative monoclonal antibodies in clinical trials focused on the treatment and diagnosis of cancer. The company is pursuing multiple clinical programs in cancer with its lead immunotherapy candidate bavituximab while seeking a partner to further advance its novel brain cancer agent Cotara®. Peregrine also has in-house cGMP manufacturing capabilities through its wholly-owned subsidiary Avid Bioservices, Inc. ( http://www.avidbio.com ), which provides development and biomanufacturing services for both Peregrine and third-party customers. Additional information about Peregrine can be found at http://www.peregrineinc.com .
Safe Harbor *snip*
Contact: Christopher Keenan or Jay Carlson, Peregrine Pharmaceuticals, (800) 987-8256, info@peregrineinc.com
= = = = = = = = = = = = = = = = = = = = =
12-30-13: "Peregrine Pharmaceuticals Initiates SUNRISE Pivotal Phase III Clinical Trial of Bavituximab in Second-Line Non-Small Cell Lung Cancer”
· Company Launches http://www.SunriseTrial.com to Assist Patients and Physicians
http://ir.peregrineinc.com/releasedetail.cfm?ReleaseID=816379
TUSTIN, 12/30/13: Peregrine Pharmaceuticals, Inc. (NASDAQ: PPHM), today announced the opening to enrollment of its SUNRISE trial at leading oncology centers in the United States. SUNRISE is a pivotal Phase III clinical trial comparing the company's investigational immunotherapy bavituximab plus the chemotherapy docetaxel against placebo plus the chemotherapy docetaxel in patients with second-line non-small cell lung cancer (NSCLC). This trial will enroll approximately 600 patients from more than 100 medical centers worldwide.
"The design of the SUNRISE trial was based on the compelling Phase II data [6-3-13/ASCO’13: Final Data Ph.II 2L/NSCLC http://tinyurl.com/my8qxw7 ] demonstrating encouraging improvement in overall survival in patients with second-line NSCLC. Furthermore, peer-reviewed published data support that bavituximab and docetaxel share highly compatible mechanisms of action that we believe hold promise for improved patient outcomes," (1-6) said Joe Shan, MPH, VP of Clinical & Regulatory Affairs at Peregrine. "We believe harnessing the body's natural immune system to fight cancer will be an integral part to conquering this deadly disease. Multiple peer-reviewed published data support bavituximab's immunotherapy mechanism of action whereby the targeted monoclonal antibody blocks an immune checkpoint responsible for immune suppression at the local tumor environment, thereby allowing the immune system to recognize and fight this deadly disease."
"This is a significant milestone for the company as patients with advanced non-small cell lung cancer who have progressed on a prior treatment have few therapeutic options, and new approaches to managing their disease are in demand," said Steven King, CEO of Peregrine. "As part of our plan to provide patient and physicians with the necessary information regarding this trial, today we launched the http://SunriseTrial.com web portal to serve as a gateway for trial parameters and additional resources. We anticipate that in executing our global plan we can enroll the majority of patients in this trial from the United States and Western Europe."
SUNRISE ("Stimulating ImmUne RespoNse thRough BavItuximab in a PhaSE III Lung Cancer Study") is a Phase III, global, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the safety, tolerability and efficacy of bavituximab plus docetaxel in patients with second-line NSCLC. The trial will evaluate bavituximab plus docetaxel versus docetaxel plus placebo in approximately 600 patients at clinical sites worldwide. Patients with Stage IIIb/IV non-squamous, NSCLC who have progressed after standard front-line treatment are eligible for enrollment. Patients will be randomized into 1 of 2 treatment arms. All patients will receive up to six 21-day cycles of docetaxel (75 mg/m2) plus weekly infusions of either bavituximab (3mg/kg) or placebo until progression of toxicity. The primary endpoint of the trial will be overall survival. For additional information about the SUNRISE trial please visit http://www.SunriseTrial.com or ClinicalTrials.gov using Identifier NCT01999673. [ http://www.clinicaltrials.gov/ct2/show/NCT01999673 ]
About Peregrine Pharmaceuticals, Inc.
Peregrine Pharmaceuticals, Inc. is a biopharmaceutical company with a portfolio of innovative monoclonal antibodies in clinical trials focused on the treatment and diagnosis of cancer. The company is pursuing multiple clinical programs in cancer with its lead immunotherapy candidate bavituximab while seeking a partner to further advance its novel brain cancer agent Cotara®. Peregrine also has in-house cGMP manufacturing capabilities through its wholly-owned subsidiary Avid Bioservices, Inc. ( http://www.avidbio.com ), which provides development and biomanufacturing services for both Peregrine and third-party customers. Additional information about Peregrine can be found at http://www.peregrineinc.com .
Safe Harbor *snip*
REFERENCES [See: http://www.peregrineinc.com/pipeline/bavituximab-oncology.html & http://www.peregrineinc.com/technology/bavituximab-oncology.html ]
1. Yin et al. “Phosphatidylserine-Targeting Antibody Induces M1 Macrophage Polarization and Promotes Myeloid-Derived Suppressor Cell Differentiation”, Cancer Immunology Research. 2013 Oct 1 (4):256-68.
2. Fabbri et al. “Sequential events of apoptosis involving docetaxel, a microtubule-interfering agent: a cytometric study” BMC Cell Biol. 2006 Jan 26;7:6.
3. Gong et al. “Measuring response to therapy by near-infrared imaging of tumors using a phosphatidylserine-targeting antibody fragment” Mol Imaging. 2013 Jun 1;12(4):244-56.
4. Huang et al. “A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice” Cancer Res. 2005 May 15;65(10):4408-16.
5. Kodumudi et al. “A Novel Chemoimmunomodulating Property of Docetaxel: Suppression of Myeloid-Derived Suppressor Cells in Tumor Bearers” Clin Cancer Res 2010;16:4583-94.
6. Hodge et al. “Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death”. Int. J. Cancer: 2013; 133(3):624-36.
CONTACT:
Christopher Keenan or Jay Carlson - Peregrine Pharmaceuticals, (800) 987-8256, info@peregrineinc.com
= = = = = = = = = = = = = =
BAVITUXIMAB PHASE III TRIAL: ( http://www.SunriseTrial.com )
A. Phase III Bavi+Doce vs. 2nd-Line NSCLC "SUNRISE" (randomized, double-blind, placebo-ctl'd, n=582)
Protocol: http://www.clinicaltrials.gov/ct2/show/NCT01999673 (Start=Dec2013 Est PrimaryComp=Dec2016)
1-6-14: FDA grants FAST TRACK status to Bavi in 2ndLine NSCLC http://tinyurl.com/l799ukk
12-30-13: Pivotal Ph.3 ‘SUNRISE’ NSCLC Trial Initiated (n=~600, sites=~100) http://tinyurl.com/kdjb9qz
5-20-13: FDA Approves Bavituximab Ph.III Design for 2L/NSCLC; 600-pt trial to begin by y/e’13 http://tinyurl.com/n3dxtm6
...S.King: “We will now focus on starting the Ph.III trial while continuing ongoing partnering discussions.”
…R.Garnick: “This was a highly collaborative effort with the FDA; this trial, when combined with Bavi’s supporting data to date, could be sufficient to support a future BLA submission."
= = = = = = = = = = = = = = = = = = = = = = = = =
PRIOR (2010-2011) PHASE II 2nd-LINE NSCLC BAVI+DOCE TRIAL (n=121):
F. LEAD IND: Phase IIb Bavi+Doce vs. 2nd-Line NSCLC (randomized, double-blinded, placebo-ctl'd, n=120, 'registrational')
Protocol: http://clinicaltrials.gov/ct2/show/NCT01138163 (24 U.S. + 15 India + 2 RepGA + 7 RussianFED + 5 Ukraine = 53 as of 8-12-11)
Enrolled Oct2010 - Oct2011 at 40 global sites (per J.Shan 9-7-12 webcast ( http://tinyurl.com/8cn87la )
8-2012: Compare Bavi+Doce's MOS=11.7mos (Bavi/3mg) to the 4 Curr-Approved 2Line/NSCLC Drugs http://tinyurl.com/cgnkvpa
• Taxotere/docetaxel => MOS=6.3mos (meta-analysis of 5 trials, 865 pts)
• Altima/pemetrexed => No diff. vs. Docetaxel (Ph.3 non-inferiority vs. Doce, 571 pts)
• Tarceva/erlotinib => MOS=5.3mos (TITAN Ph.III n=424 trial - see http://tinyurl.com/8w8lo93 )
• Iressa/gefitinib => "Iressa does not improve OS"
6-3-13/ASCO’13: Final Data Ph.II 2L/NSCLC http://tinyurl.com/my8qxw7
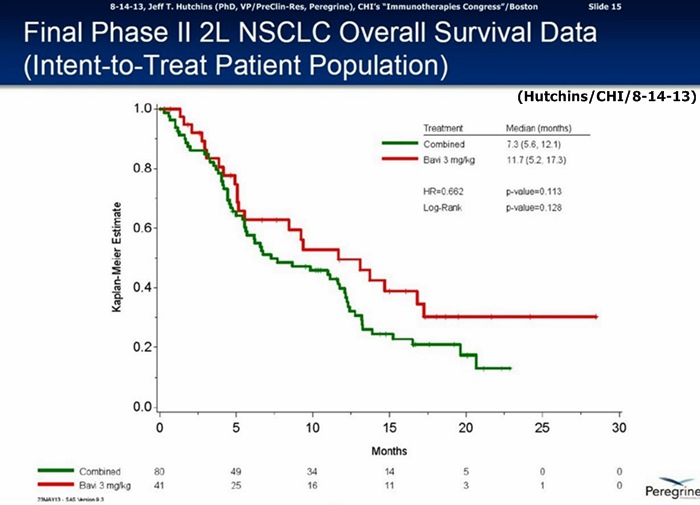
…60% improvement in MOS: Bavi/3mg=11.7mos. vs. 7.3mos. for CTL-arm(combined Bavi/1mg + DoxyOnly arms), HR=.662, P=.113
5-20-13: FDA Approves Bavituximab Ph.III Design for 2L/NSCLC; 600-pt trial to begin by y/e’13 http://tinyurl.com/n3dxtm6
...S.King: “We will now focus on starting the Ph.III trial while continuing ongoing partnering discussions.”
…R.Garnick: “This was a highly collaborative effort with the FDA; this trial, when combined with Bavi’s supporting data to date, could be sufficient to support a future BLA submission."
2-19-13: Topline Data Update from 2nd-Line NSCLC Trial after data discrepancies review http://tinyurl.com/ansqcea
…60% improvement in MOS: 3mg=11.7mos. vs. 7.3mos. for CTL-arm(combined 1mg & Doxy+placebo arms), HR=.73, p=.217
6-5-13: FTM's table of MOS data in 15 prior Doxy 2nd-Line NSCLC trials (Bavi's 60% MOS Improvement is Tops) http://tinyurl.com/m886ctb
1-25-13: MLV's George Zavoico recaps 2ndLine/NSCLC data errors & current status of PPHM's review http://tinyurl.com/b9u4pk8
...GZ: "This means that patients randomized into the high dose arm were administered Bavi correctly, whereas some of the patients in the placebo arm were administered low dose Bavi and some in the low dose Bavi arm were administered placebo. More importantly, the findings suggested that the MOS of 13.1 mos. in the high dose arm was likely to be valid. Even by historical measures, this is a remarkable result, since docetaxel's product insert lists the MOS of NSCLC patients receiving this widely used drug as 2nd-Line therapy in 2 trials as 5.7 & 7.5 mos. In effect, adding Bavi doubled the MOS. In our view, this was an extraordinary stroke of luck. If the high dose arm had been affected by the coding discrepancy, Peregrine would have been in a completely different & unfortunate position… Moreover, Peregrine must determine how best to present its case to the FDA. Will the historical controls be sufficient to justify moving Bavi into a Phase III pivotal trial, or will Peregrine have to pool the results of the placebo & low-dose arms and use that as a comparator to argue for moving ahead? A simple average of the placebo & low-dose arms results in a new control MOS of about 8.4 mos., still several months less than that of the high dose arm. This quick analysis results in about a 5-mo. survival advantage, a substantial prolongation for patients with second-line NSCLC and likely to justify moving Bavi into a pivotal Phase III trial in 2013, in our view."
1-7-13 PPHM PR - Review Update: "discrepancies are isolated to the placebo and 1 mg/kg arms; no evidence of discrepancies in the 3 mg/kg arm… Peregrine is taking a very conservative approach toward analyzing the results by combining the placebo & 1mg/kg arms into one treatment arm (control arm), and comparing to the 3mg/kg arm. This analysis indicates that the 3 mg/kg arm continues to show favorable TRR's, PFS, and OS over the new combined control arm. Peregrine expects to announce more detailed results from the analysis in the near term when it is completed." http://tinyurl.com/asup54d
9-24-12: Major Discrepancies found in 2nd-Line NSCLC Ph.2B Treatment Group Coding by Indep. Third-Party Vendor http://tinyurl.com/8r9zcqy
…"Investors should not rely on clinical data that the company disclosed on or before Sept. 7, 2012 from its Ph.2 Bavi trial in patients with 2nd-Line NSCLC or any presentations or other documents related to this Ph.2 trial."
9-24-12: Peregrine sues CSM Over Bavi Ph.2B 2nd-Line NSCLC Clinical Trial Mix-Up http://tinyurl.com/8fpgngu
…CSM = Clinical Supplies Management Inc., Fargo ND http://www.csmondemand.com
...1-17-13: Peregrine's lawsuit against CSM for "breach of contract & negligence" SERVED http://tinyurl.com/a7zrgys
…9-10-12 CEO Steve King, QtlyCC ( http://tinyurl.com/8nkwrml )
……"These are truly remarkable results (statistically doubling MOS) that are not only great for the pgm… but also great news for the NSCLC patients in the trial…"
…9-10-12 Robert Garnick (Head/Reg), QtlyCC ( http://tinyurl.com/8nkwrml )
……"The NSCLC data we announced 9-7-12 has far exceeded our expectations, and I hope that you're as excited as I am with bavituximab's potential. I feel strongly that Peregrine should be recognized for having the corporate courage to conduct the rigorous, randomized placebo-controlled Phase II trial that provided these robust data and that provide the basis for us to plan for a pivotal Phase III program."
...9-7-12: PPHM Press Release about Dr. Gerber's plenary at ASTRO/Thoracic/Chicago: http://tinyurl.com/96wrrso
…"The interim data showed a statistically significant improvement in OS (Hazard Ratio 0.524, p-value .0154) and a doubling of MOS (12.1/13.1mos. vs. 5.6mos.) in the Bavituximab-containing arms compared to the [Docetaxel] ctl-arm."
......VP Joe Shan's 15min. Webcast & Slideshow recapping Dr. David Gerber's 9-7-12 ASTRO/Chicago Plenary: http://tinyurl.com/96wrrso
…8-15-12 CEO Steve King, Wedbush/NYC ( http://tinyurl.com/8mhrtld )
......"As we're sitting here today, we have still not reached the # of events for MOS in either of the Bavituximab arms - and, in fact, we still have patients that are on treatments." Q&A: "it's going to be a very positive MOS result, it's just a matter now of magnitude."
…7-16-12 CEO Steve King, QtlyCC ( http://tinyurl.com/cs7spbz )
......"The strength of this 2nd-Line NSCLC data (esp. MOS trends) in this large area of high unmet medical need has also sparked a surge in partnering discussions that has included over 15 in-person partnering meetings since that time with major players in oncology, with all discussions ongoing and addl. parties showing interest. Our goal for the program is to position ourselves, along with a potential partner, to initiate Ph3 by mid-2013, which means an EOP2 meeting by yr-end'12. It would be ideal to have a partner on board to participate in the EOP2 meeting, and we have communicated this to interested parties and they agree."
…7-16-12 Robert Garnick (Head/Reg), QtlyCC ( http://tinyurl.com/cs7spbz )
……"We've been working very hard and very actively on the next steps in our Bavi 2nd-Line NSCLC pgm, given the favorable data that we've seen. As you can imagine, with data like this, there are many things that we need to consider. One consideration is that, should the data continue to trend the way it is, particularly in survival, this opens a door for potential discussions around a pathway for Accelerated Approval. At this point, all options are being considered, with Peregrine working towards the most efficient path forward from a regulatory standpoint." Q&A: "…all in all, I think the data is extremely compelling and I think it makes a really good case. Certainly, I think, I've seen a lot of Ph2 & Ph3 data, and this is as compelling Ph2 data as I've ever seen. So, I'm very comfortable proposing a meeting with the FDA for Q4'12."
…7-12-12 CEO Steve King, JMP-Conf/NYC ( http://tinyurl.com/csdclwb )
……"Re: 2nd-Line/NSCLC trial, the most thrilling thing is the fact that, even though we'd reached MOS for the ctl-arm(Doce) at end of Apr'12 of LESS THAN 6MOS, the majority of patients are still alive (today) in both Bavi arms, and we expect that to continue for some period of time still. Ph3 planning is underway already; our goal is to start this Ph3 by mid'13, meaning an EOP2 meeting with the FDA in Q4'12; our goal is to bring a partner on board, ideally in time for that EOP2 meeting, certainly before the beg. of the Ph3 trial."
…5-21-12: TopLine data n=117 for Bavi/3mg+Doce arm: ORR=17.9%/PFS=4.5mos (vs. CTL 7.9%/3mos) http://tinyurl.com/73aeyxj
......Importantly, MOS for CTL-arm "< 6 mos", but not yet reached in both Bavi arms.
...10-6-11: Enrollment complete. http://tinyurl.com/3m9re39
...7-14-11/CC: Enrollment was taking longer than expected; have amended protocol; expanding to ~45 sites, expect enroll. comp. "early in Q4/2011", data unblinding 1H'12. http://tinyurl.com/6k6y2as
…3-17-10/Roth, CEO S.King: "We refer to this trial as a Registrational Phase II Study, because we believe that if we have results anywhere near approaching what we saw in the earlier [India] study, it could be a conduit for Accelerated Approval."
...6-4-10: Ph.2b randomized reg. trial Open for enrollment: http://tinyurl.com/25v22qk
……"up to 120 refractory patients at ~30 clinical sites; goal: fully-enroll by mid'11, topline data by y/e'11."
Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
FEATURED Cannabix Technologies and Omega Laboratories Inc. Advance Marijuana Breathalyzer Technology - Dr. Bruce Goldberger to Present at Society of Forensic Toxicologists Conference • Sep 24, 2024 8:50 AM
FEATURED Integrated Ventures, Inc Announces Strategic Partnership For GLP-1 (Semaglutide) Procurement Through MedWell USA, LLC. • Sep 24, 2024 8:45 AM
Avant Technologies Accelerates Creation of AI-Powered Platform to Revolutionize Patient Care • AVAI • Sep 24, 2024 8:00 AM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM






