Friday, May 19, 2017 3:09:38 PM
May18-21: ICHC’17, 15th Intl. Congress of Histo/Cyto Chemistry - from Molecules to Diseases, Turkey http://tinyurl.com/kbetmbk
...May19/12pm: Raymond Birge (Rutgers), “Phosphatidylserine Sensing by TAM Receptors Regulates AKT-dependent Chemo-Resistance & PD-L1 Expression”
Jun2-6: ASCO 2017, Chicago https://am.asco.org See: http://tinyurl.com/kkpqvra
PPHM Exhibiting – booth #13124 (next to Moffitt CC)
#1: 6-3-17 1:15-4:45pm, #11603, “Prelim. Correlative Analysis of PD-L1 Expression from the Sunrise Study” (Sunrise Biomarker #4)
...11 Authors: LEAD: Nikoletta Lea Kallinteris (PPHM); SENIOR: Cliff Hoyt (PerkinElmer, "Technology Strategist")
#2: #e23091 (pub.only): “Effect Of Bavituximab In Combination With Nivolumab on Tumor Immune Response in a 3D Ex Vivo System of Lung Cancer Patients”
...Authors: LEAD is from Nilogen Oncosystems, SENIOR from Moffitt w/Scott Antonio and PPHM co-authors.
**See ASCO 2017 DETAILS BELOW**
Jun18-20: ICDS’17, Annual ICDS Conf. on Cell Death in Development & Disease, Israel http://www.weizmann.ac.il/conferences/CDDD2017
...Jun20 11:30-12:00pm: Raymond Birge (Rutgers), “Regulation of Efforocytosis by Phosphatidyserine Receptor”
Jun19-22/Avid Booth #1411: BIO Intl. Convention, SanDiego http://www.convention.bio.org/2017
~Jul13: FY'17Q4 (fye 4-30-17) Financials & Conf. Call - http://ir.peregrineinc.com/events.cfm
Aug21-25/Avid Booth #403: CHI's 9th Annual BioProcessing Summit, Boston http://www.bioprocessingsummit.com
~Sep7: FY'18Q1 (qe 7-31-17) Financials & Conf. Call - http://ir.peregrineinc.com/events.cfm
Sep25-28/Avid Exhibiting: Informa’s BPI’17: BioProcess Intl. Conf. & Exhibition, Boston https://lifesciences.knect365.com/bioprocessinternational
~Oct12: Peregrine's Annual Shareholder’s Meeting (2016 attendee reports: http://tinyurl.com/jx7ouay )
.
.
======================ASCO’17 DETAILS:
ASCO’17: PPHM’s 2 Abstracts (11603/June3, e23091/pub.only)
#1: 6-3-17 poster #11603 “Prelim. Corr. Analysis of PD-L1/Expression from SUNRISE” has 11 authors: Lead author is PPHM’s Nikoletta L.Kallinteris, and Senior Author is PerkinElmer’s Technology Strategist Cliff Hoyt, whose function at PKI is to "help identify clinical opportunities that can be addressed through new technologies & capabilities, including forming collaborations & partnerships with academic & industrial institutions.”
#2: Pub. Only (Jrnl Clin. Oncology), “Effect Of Bavituximab In Combination With Nivolumab on Tumor Immune Response in a 3D Ex Vivo System of Lung Cancer Patients”. Senior author is Moffitt’s Soner Altiok (with co-author Moffitt’s Scott Antonia), and Lead author is Nilogen Oncosystems’ Melanie Mediavilla-Varela (see http://www.nilogen.com ).
Jun2-6: “ASCO 2017”, Chicago https://am.asco.org
...PPHM Exhibiting – booth #13124 (next to Moffitt CC)
Abstracts: http://abstracts.asco.org (rel. 5-17-17 5:00pmET)
PPHM #1: 6-3-17 1:15-4:45pm, Abstract #11603, Track: Tumor Biology
“Preliminary Correlative Analysis of PD-L1 Expression from the Sunrise Study” (poster board #303)
Note: this is known Sunrise Biomarker #4 (PD-L1 Expr.)
http://abstracts.asco.org/199/AbstView_199_190902.html
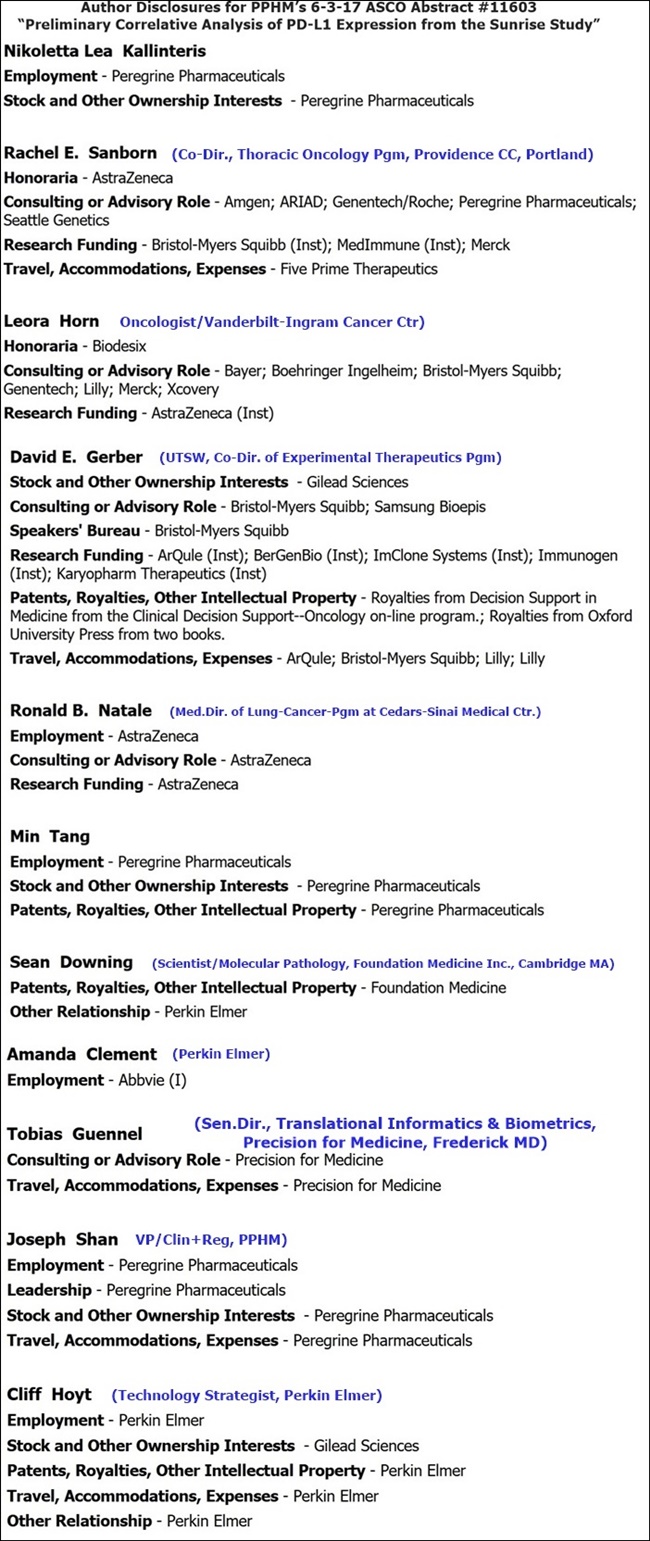
AUTHORS:
LEAD: Nikoletta Lea Kallinteris (PPHM)
Rachel E. Sanborn (Co-Dir., Thoracic Oncology Pgm, Robert W. Franz Cancer Res. Center, Earle A. Chiles Res. Inst., Providence CC, Portland; Honoraria=AstraZeneca; Consulting=Amgen/Ariad/DNA/PPHM/SeaGen; Funding=BMS/MedImmune/Merck)
Leora Horn (Oncologist/Vanderbilt-Ingram CC; Honoraria=Biodesix; Funding=AZN)
David E. Gerber (UTSW)
Ronald B. Natale (Med.Dir/Lung-Cancer-Pgm/Cedars-Sinai Medical Center; consult/funding=AstraZeneca)
Min Tang (PPHM)
Sean Downing (Scientist/Molecular Pathology, Foundation Medicine Inc., Cambridge MA) http://www.foundationmedicine.com ; disclosure=PerkinElmer)
Amanda Clement (Scientist/PerkinElmer; disclosure=Abbvie)
Tobias Guennel (Sen.Dir., Translational Informatics & Biometrics, Precision for Medicine, Frederick MD https://www.precisionmedicinegrp.com/pfm )
Joseph Shan (PPHM, VP/Clin+Reg)
SENIOR AUTHOR: Cliff Hoyt (Oncology Fellow/PerkinElmer)
…Note (Nov2016):
“Mr. Hoyt is a Technology Strategist in the area of oncology, helping to identify clinical opportunities that can be addressed through new technologies & capabilities. This includes forming collaborations & partnerships with academic & industrial institutions.”
http://www.sciencemag.org/custom-publishing/webinars/multiplexed-immunohistochemistry-illuminating-tumor-microenvironment
Interesting: 5-1-15/1hr. Webinar Video: Clifford Hoyt (PerkinElmer) & Bernard Fox (Providence CC, Portland) - “Cancer immunology: Charting The Course Forward For Immunoprofiling” (Dr. Hoyt speaks at 31:30)
- - - - - - - - - -
**Peregrine: N.Kallinteris (Lead Author), M.Tang, Joe Shan
**Robert W. Franz CC, Earle A. Chiles Res. Inst., Providence CC, Portland: Rachel E. Sanborn
**Vanderbilt Univ. MC, Nashville: Leora Horn
**UTSW-MC/Dallas: David Gerber
**Cedars-Sinai Outpatient CC, Los Angeles: Ronald B. Natale
**PerkinElmer, Inc., Hopkinton, MA: Amanda Clement, Clifford Hoyt (Senior Author)
**Precision Medicine, Frederick, MD: Tobias Guennel
- - - - - - - - - - - - - -
ABSTRACT - BACKGROUND:
SUNRISE, a global, double-bind, Phase III trial of docetaxel (D) + bavituximab (B) or D plus placebo (P) in previously treated non-squamous NSCLC, demonstrated similar overall survival (OS) in both treatment arms. Biomarkers including pre-treatment PD-L1 expression are being retrospectively assessed in on-going exploratory analyses.
METHODS:
Archival tissue obtained at the time of diagnosis was requested but not required in the SUNRISE trial. FFPE slides were stained with a panel of lymphoid cell markers: CD3+, CD8+, FoxP3+, PD-L1+, CD163+, CK+ and DAPI using a 6-plex quantitative immunohistochemistry (IHC) assay (OPAL, PerkinElmer, Hopkinton, MA). Baseline PD-L1 expression was retrospectively scored on tumor cells (TC) as a percentage of PD-L1 expressing tumor cells: TC3>=50%, TC2>=5% and < 50%, TC1>=1% and < 5%, and TC0 < 1%. Cox regression models for PD-L1 IHC subgroup populations were used for correlation with OS.
RESULTS:
In the subset of patients with available diagnostic biopsies (110 out of 597 randomized patients), the prevalence of PD-L1 expression was 5% for TC3, 18% for TC2/3, 35% for TC1/2/3, 65% for TC0. Median OS (mOS) of the D+B arm is 11.5 months (TC0, < 1%) and 6.0 months (TC1/2/3, >=1%) with HR 0.38 (95% CI, 0.19-0.76); p-value = 0.004. mOS of the D+P arm is 11.1 months (TC0, < 1%) and 10.4 months (TC1/2/3, >=1%) with HR 0.93 (95% CI, 0.47-1.87); p value = 0.844.
CONCLUSIONS:
Baseline PD-L1 expression in a subset of SUNRISE patients demonstrated that PD-L1 expression (TC0) was associated with a significantly prolonged OS compared to positive PD-L1 expression (TC1/2/3) in patients receiving D+B. No difference in OS was observed in the D+P group by PD-L1 expression. These observations are consistent with the hypothesis that bavituximab may demonstrate more effect in PD-L1 negative or low expressing “immune cold” tumors.
Clinical trial info: https://www.clinicaltrials.gov/ct2/show/NCT01999673
= = = = = = = = = = = = = =
PPHM #2 (PUB. ONLY, NOT PRESENTED AT MEETING - J Clin Oncol 35/2017/Suppl)
Category: Tumor Biology, Sub-cat: Immunobiology
#e23091: “Effect Of Bavituximab In Combination With Nivolumab on Tumor Immune Response in a 3D Ex Vivo System of Lung Cancer Patients”
http://abstracts.asco.org/199/AbstView_199_193973.html
AUTHORS:
Nilogen Oncosystems: Melanie Mediavilla-Varela (LEAD), Melba Marie Page, Jenny Kreahling http://www.nilogen.com ”Bringing the complex tumor microenvironment to life with 3D ex vivo assays”
Moffitt Cancer Center: Scott J. Antonia, Soner Altiok (SENIOR AUTHOR) https://moffitt.org
Peregrine: Bruce D Freimark, Joseph Shan, Nikoletta Lea Kallinteris
- - - - - - - - - -
Dr. Scott J. Antonia, MD/PhD serves as Medical & Scientific Advisor of Peregrine Pharmaceuticals. He advises Cellular Biomedicine Group on immuno-oncology. Dr. Antonia serves as Chair of Thoracic Oncology Dept. and Leader of the Immunology Pgm. at the H. Lee Moffitt Cancer & Research Institute in Tampa. Using his molecular biology & cellular background in the dev. of immunotherapeutic strategies for the treatment of cancer patients, he has developed strategies designed to thwart the immunosuppressive mechanisms used by tumors to evade T-cell mediated rejection...” Honoraria/Advisor=AstraZeneca/Boehringer Ingelheim/BMS/Merck http://tinyurl.com/klnbfkh
Dr. Soner Altiok, MD/PhD - ”As a Cytopathologist at Moffitt CC, my focus is on applying techniques of cell biology & proteomics to the field of cytopathology to identify novel diagnostic and prognostic biomarkers.” Honoraria/Advisor/ExpertTestamony=Genentech https://moffitt.org/research-science/researchers/soner-altiok/
ABSTRACT
BACKGROUND:
Bavituximab is a chimeric monoclonal antibody that targets the membrane phospholipid phosphatidylserine (PS) exposed on endothelial cells and cancer cells in solid tumors. Our previous studies showed that bavituximab enhances the activation of CD8+ TILs that correlates with increased cytokine production by lymphoid and myeloid cells in lung cancer with low PD-L1 expression suggesting that the interruption of the PD-1/PD-L1 axis by nivolumab may enhance the bavituximab effect in tumors.
METHODS:
Fresh tumor tissues obtained from consented patients with NSCLC at the time of surgical resection were utilized in a 3D ex vivo tumor miscrosphere assay, where 3D tumor microspheres were treated with bavituximab or nivolumab alone or in combination at 10 mg/ml for 36 hours. At the end of the treatment, a multiplex human cytokine assay was used to simultaneously analyze the differential secretion of cytokines, including human IFNg, in culture media as a surrogate of TIL activation. In addition, a gene expression analysis using a NanoString platform containing probes to quantitate 770 immune function genes.
RESULTS:
Preliminary results indicate the combination treatment with bavituximab & nivolumab led to increased expression of genes involved in M1 polarization of tumor associated macrophages in a subpopulation of lung tumors that closely correlated with release of cytokines such as MIP1b (CCL4) which is a chemoattractant for natural killer cells, monocytes and a variety of other cells involved in tumor immune response.
CONCLUSIONS:
This lung patient derived ex-vivo approach indicates that bavituximab in combination with nivolumab may enhance immune response. This response likely involves M1 polarization of tumor associated macrophages and suggests potential clinical implications in the treatment of lung cancer.

=========================
NOTE: 4 Known Sunrise Biomarkers thru 5-2017(ASCO): B2GPI, Complement+IL10, IFN-y, PD-L1 Expr.

Recent CDMO News
- Avid Bioservices to Participate in Craig-Hallum Bioprocessing Conference • GlobeNewswire Inc. • 09/12/2024 08:05:27 PM
- Form 10-Q - Quarterly report [Sections 13 or 15(d)] • Edgar (US Regulatory) • 09/09/2024 08:43:56 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 09/09/2024 08:19:30 PM
- Avid Bioservices Reports Financial Results for First Quarter Ended July 31, 2024 • GlobeNewswire Inc. • 09/09/2024 08:05:31 PM
- U.S. Futures Rise Amid Inflation Report Anticipation; Oil Prices Climb on Hurricane Threat and Supply Concerns • IH Market News • 09/09/2024 10:09:14 AM
- Avid Bioservices to Report Financial Results for First Quarter of Fiscal Year 2025 After Market Close on September 9, 2024 • GlobeNewswire Inc. • 09/03/2024 08:05:20 PM
- Form 8-K - Current report • Edgar (US Regulatory) • 08/29/2024 08:30:10 PM
- Form ARS - Annual Report to Security Holders • Edgar (US Regulatory) • 08/28/2024 08:34:04 PM
- Form DEFA14A - Additional definitive proxy soliciting materials and Rule 14(a)(12) material • Edgar (US Regulatory) • 08/28/2024 08:32:18 PM
- Form DEF 14A - Other definitive proxy statements • Edgar (US Regulatory) • 08/28/2024 08:30:28 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:50:20 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/16/2024 11:48:19 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/15/2024 08:40:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/12/2024 08:30:04 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:39 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:02:27 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:22 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:01:05 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:54 PM
- Form 4 - Statement of changes in beneficial ownership of securities • Edgar (US Regulatory) • 07/11/2024 09:00:45 PM
- Form 144 - Report of proposed sale of securities • Edgar (US Regulatory) • 07/11/2024 12:23:26 AM
- Avid Bioservices Reports Financial Results for Fourth Quarter and Fiscal Year Ended April 30, 2024 • GlobeNewswire Inc. • 07/02/2024 08:05:04 PM
- Avid Bioservices to Report Financial Results for Quarter and Fiscal Year Ended April 30, 2024, After Market Close on July 2, 2024 • GlobeNewswire Inc. • 07/01/2024 11:00:21 AM
- Avid Bioservices Earns Committed Badge from EcoVadis for Sustainability Performance • GlobeNewswire Inc. • 05/23/2024 12:05:46 PM
- Avid Bioservices to Participate at Upcoming Investor Conferences • GlobeNewswire Inc. • 05/07/2024 08:05:11 PM
VHAI - Vocodia Partners with Leading Political Super PACs to Revolutionize Fundraising Efforts • VHAI • Sep 19, 2024 11:48 AM
Dear Cashmere Group Holding Co. AKA Swifty Global Signs Binding Letter of Intent to be Acquired by Signing Day Sports • DRCR • Sep 19, 2024 10:26 AM
HealthLynked Launches Virtual Urgent Care Through Partnership with Lyric Health. • HLYK • Sep 19, 2024 8:00 AM
Element79 Gold Corp. Appoints Kevin Arias as Advisor to the Board of Directors, Strengthening Strategic Leadership • ELMGF • Sep 18, 2024 10:29 AM
Mawson Finland Limited Further Expands the Known Mineralized Zones at Rajapalot: Palokas step-out drills 7 metres @ 9.1 g/t gold & 706 ppm cobalt • MFL • Sep 17, 2024 9:02 AM
PickleJar Announces Integration With OptCulture to Deliver Holistic Fan Experiences at Venue Point of Sale • PKLE • Sep 17, 2024 8:00 AM







